A) N2O
B) NF3
C) H2S
D) SeO3
E) CH3Cl
Correct Answer

verified
Correct Answer
verified
True/False
Pi bonds are covalent bonds in which the electron density is concentrated above and below the plane of the nuclei of the bonding atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement about orbital hybridization is incorrect?
A) The carbon atom in CH4 is sp3 hybridized.
B) The carbon atom in CO2 is sp hybridized.
C) The nitrogen atom in NH3 is sp2 hybridized.
D) sp2 hybrid orbitals are coplanar,and at 120° to each other.
E) sp hybrid orbitals lie at 180° to each other.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules is polar?
A) CH4
B) CHBr3
C) F2
D) CBr4
E) CO2
Correct Answer

verified
Correct Answer
verified
Short Answer
Use the VSEPR model to predict the molecular geometry of H3O+ (hydronium ion).
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a polyatomic ion having the general formula AB3n±,where A is an atom from Group 6A,B is an atom from Group 7A,and the ionic charge is n±,what charge must the ion have in order to yield a molecular geometry that is trigonal pyramidal?
A) 3-
B) 1-
C) 1+
D) 3+
E) 5+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the most reasonable prediction for the three F-Br-F bond angles in BrF3?
A) 90°,90°,and 180°
B) 86°,94°,and 180°
C) 86°,86°,and 172°
D) 94°,94°,and 172°
E) 120°,120°,and 120°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has a nonzero dipole moment?
A) BeCl2
B) SF2
C) KrF2
D) CO2
E) CCl4
Correct Answer

verified
Correct Answer
verified
Essay
In one sentence state the basic principle of valence bond theory.
Correct Answer

verified
A covalent bond forms when the...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
According to the VSEPR model,the predicted molecular geometry of SiCl4 is
A) linear.
B) trigonal planar.
C) bent.
D) tetrahedral.
E) trigonal pyramidal.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is true for carbon monoxide?
A) CO has a short CO bond.
B) CO has a zero dipole moment.
C) The bond energy in CO is low.
D) The atoms in CO cannot obey the octet rule.
E) All of the are true about CO.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of ClO3F as predicted by the VSEPR model? 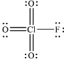
A) trigonal pyramidal
B) square planar
C) square pyramidal
D) tetrahedral
E) octahedral
Correct Answer

verified
Correct Answer
verified
Essay
In not more than two sentences,explain when and why chemists make use of the concept of hybridization.
Correct Answer

verified
Chemists postulate hybridizati...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What is the predicted O-C-O bond angle in CO2?
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the VSEPR model,a molecule with the general formula AB2 with two lone pairs on the central atom will have a _____ molecular geometry.
A) linear
B) bent
C) trigonal planar
D) tetrahedral
E) seesaw
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the predicted molecular geometry of the CH4 molecule according to the VSEPR model?
A) tetrahedral
B) trigonal pyramidal
C) trigonal planar
D) square planar
E) seesaw
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of NOCl as predicted by the VSEPR model? 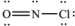
A) linear
B) trigonal planar
C) bent
D) tetrahedral
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to molecular orbital theory,what is the bond order in the O2+ ion?
A) 5.5
B) 5
C) 4
D) 2.5
E) 1.5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the molecular geometry and polarity of the CS2 molecule.
A) linear,polar
B) linear,nonpolar
C) tetrahedral,nonpolar
D) bent,nonpolar
E) bent,polar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of As in the AsF4- ion?
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 137
Related Exams