Correct Answer

verified
Correct Answer
verified
Multiple Choice
What product forms at the anode during the electrolysis of molten NaBr?
A) Na+(l)
B) Na(l)
C) Br-(l)
D) Br3-(l)
E) Br2(g)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is E°cell for the following reaction? 2Fe2+(aq) + Cd2+(aq) → 2Fe3+(aq) + Cd(s) Fe3+(aq) + e- →Fe2+ (aq) E° = 0.77 V Cd2+(aq) + 2e- → Cd(s) E° = -0.40 V
A) -0.37 V
B) 0.37 V
C) -1.17 V
D) 1.17 V
E) None of these choices is correct
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A voltaic cell consists of a Mn/Mn2+ electrode (E° = -1.18 V) and a Fe/Fe2+ electrode (E° = -0.44 V) .Calculate [Fe2+] if [Mn2+] = 0.050 M and Ecell = 0.78 V at 25°C.
A) 0.040 M
B) 0.24 M
C) 1.1 M
D) 1.8 M
E) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is incorrect?
A) At equilibrium,Ecell = 0.
B) E > 0 for a spontaneous process.
C) E = 0 for a spontaneous process.
D) ΔG < 0 for a spontaneous process.
E) ΔG = 0 at equilibrium.
Correct Answer

verified
Correct Answer
verified
Short Answer
___________ - _________ fuel cells provide electrical power and drinking water for space flights.
Correct Answer

verified
Correct Answer
verified
True/False
In a fuel cell,an external source of electrical power is used to drive a nonspontaneous reaction in which a fuel is produced.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the half-reaction at the cathode in a lead storage battery?
A) Pb(s) + PbO2(s) + 4H+(aq) + 2SO42-(aq) → 2PbSO4(s) + 2H2O(l)
B) PbO2(s) + 4H+(aq) + 2SO42-(aq) + 2e- → PbSO4(s) + 2H2O(l)
C) Pb(s) + SO42-(aq) → PbSO4(s) + 2e-
D) Pb(s) → Pb(s) + 2e-
E) H2(g) → 2H+(aq) + 2e-
Correct Answer

verified
Correct Answer
verified
True/False
E > 0 and ΔG < 0 for a spontaneous process.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is correct?
A) total charge = volts/coulombs
B) electrical energy = volts × coulombs
C) Faraday = coulombs/joules
D) coulombs = joules × volts
E) electrical work = Faraday's constant/Ecell
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements can be isolated by electrolysis of the aqueous salt shown?
A) Phosphorus from K3PO4(aq)
B) Sodium from NaBr(aq)
C) Aluminum from AlCl3(aq)
D) Fluorine from KF(aq)
E) Iodine from NaI(aq)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction in the lead-acid cell Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(aq) + 2H2O(l) For which E°cell = 2.04 V at 298 K.ΔG° for this reaction is
A) -3.94 × 105 kJ/mol.
B) -3.94 × 102 kJ/mol.
C) -1.97 × 105 kJ/mol.
D) -7.87 × 102 kJ/mol.
E) -7.87 × 105 kJ/mol.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following balanced redox reaction. 3CuO(s) + 2NH3(aq) → N2(g) + 3H2O(l) + 3Cu(s) Which of the following statements is true?
A) CuO(s) is the oxidizing agent and copper is reduced.
B) CuO(s) is the oxidizing agent and copper is oxidized.
C) CuO(s) is the reducing agent and copper is oxidized.
D) CuO(s) is the reducing agent and copper is reduced.
E) CuO(s) is the oxidizing agent and N2(g) is the reducing agent.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the following electrochemical cell is constructed,what voltage will be measured on the voltmeter? 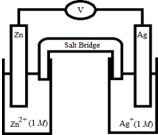 Half-Reaction E° (V) Zn2+(aq) + 2e- →Zn(s) -0.76
Ag+(aq) + e- → Ag(s) +0.80
Half-Reaction E° (V) Zn2+(aq) + 2e- →Zn(s) -0.76
Ag+(aq) + e- → Ag(s) +0.80
A) 2.36 V
B) 1.56 V
C) 0.04 V
D) -0.04 V
E) -2.36 V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is ΔG° at 200°C for the following reaction? (F = 96,500 C • mol-1) 2Na(l) + FeCl2(s)  2NaCl(s) + Fe(s) E°cell = 2.35 V
2NaCl(s) + Fe(s) E°cell = 2.35 V
A) 454 kJ/mol
B) -454 kJ/mol
C) 907 kJ/mol
D) -907 kJ/mol
E) 227 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is ΔG° for the following reaction? (F = 96,500 C • mol-1) I2(s) + 2Br-(aq) → 2I-(aq) + Br2(l) Given: I2(s) (aq) + 2e- → 2I-(aq) E°= 0.53 V Br2(l) + 2e- → 2Br-(aq) E° = 1.07 V
A) +104 kJ/mol
B) -104 kJ/mol
C) +309 kJ/mol
D) +52 kJ/mol
E) -52 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the following redox equation is balanced with the smallest whole number coefficients,what is the coefficient for nitrogen dioxide? I2(s) + HNO3(aq) → HIO3(aq) + NO2(g) + H2O(l)
A) 1
B) 2
C) 4
D) 5
E) 10
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is meant by SHE?
A) Shared half electrodes
B) Shifting half of the electricity
C) Standard hydrogen electrode
D) Small helium electrode
E) Standard Hg electrode
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the standard free-energy change for the following reaction at 25°C? (F = 96,500 C • mol-1) 2Al(s) + 3Sn4+(aq,1 M) → 2Al3+(aq,1 M) + 3Sn2+(aq,1 M) Half-Reaction Eo (V) Al3+(aq) + 3e- → Al(s) -1.66 Sn4+(aq) + 2e- →Sn2+(aq) +0.13
A) -1.79 kJ/mol
B) 1.79 kJ/mol
C) -1.04 × 103 kJ/mol
D) -3.45 × 102 kJ/mol
E) 5.18 × 102 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If ΔG° of the following reaction is -110 kJ/mol,what is E°cell? (F = 96,500 C • mol-1) A3+(aq) + 3B(s) → A(s) + 3B+(aq)
A) +0.38 V
B) -0.09 V
C) -0.38 V
D) +0.00038 V
E) +0.09 V
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 122
Related Exams