Correct Answer

verified
Correct Answer
verified
Short Answer
Based on the following energy diagram, is the reaction exothermic or endothermic? 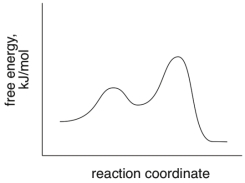
Correct Answer

verified
exothermic
Correct Answer
verified
Multiple Choice
Predict the sign of G for an endothermic reaction with an increase in entropy.
A) positive
B) negative
C) no change
D) cannot predict without additional information
Correct Answer

verified
Correct Answer
verified
Short Answer
What type of bond cleavage does the following reaction involve? 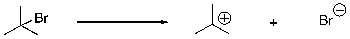
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following describes the effect of a catalyst on a reaction?
A) It lowers the free energy of the products.
B) It makes the reactants less stable.
C) It changes the equilibrium constant.
D) It lowers the energy of activation.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the sequence of curved arrows (electron movement) in the steps of the following reaction. 
A) Proton transfer, proton transfer
B) Proton transfer, loss of leaving group
C) Nucleophilic attack, proton transfer
D) Proton transfer, nucleophilic attack
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the sign of S of the following reaction. 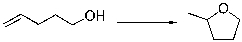
A) positive
B) negative
C) no change
Correct Answer

verified
Correct Answer
verified
Short Answer
Given the following rate law, what is the order of the reaction with respect to sodium cyanide? 
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following mechanistic steps represents a nucleophilic attack?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the most likely structure of the following cation after rearrangement?
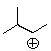
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is wrong with the following mechanism? 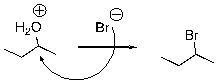
A) There is no leaving group, so there should be no arrows.
B) The arrow should be removing a proton from the H2O group.
C) An arrow is also needed to indicate the loss of the leaving group.
D) The arrow is backwards.
Correct Answer

verified
Correct Answer
verified
Essay
Draw the structure of the most likely following cation after it has rearranged. 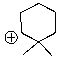
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following energy diagrams is of a reaction with one transition state?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
A
Correct Answer
verified
Short Answer
For the following reaction step, indicate which pattern of arrow pushing it represents. 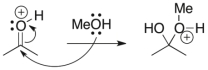
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following energy diagrams is of a reaction with one intermediate?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Essay
Identify the electrophilic site in the following molecule. 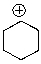
Correct Answer

verified
11eab459_a6d5_318a_acdb_7f19b0163dbd_TB3185_00
Correct Answer
verified
Multiple Choice
Consider the product of the following mechanism. How many distinct resonances would appear in the (proton-decoupled) 13C NMR spectrum for the product? 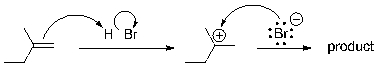
A) 2
B) 3
C) 4
D) 5
Correct Answer

verified
Correct Answer
verified
Short Answer
Predict the sign of S of the following reaction. 
Correct Answer

verified
Correct Answer
verified
Essay
Consider the following reaction. What patterns of arrow pushing are likely involved in the formation of the product? Would you expect the S for the reaction to be positive or negative? 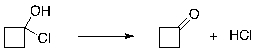
Correct Answer

verified
Proton transfer and ...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Identify the nucleophilic centers in the following molecule. 
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 110
Related Exams