A) dsp3
B) sp3
C) d2sp3
D) sp
E) sp2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the molecular-orbital description of CO,
A) the bond order is 3.
B) six molecular orbitals contain electrons.
C) there are two unpaired electrons.
D) the highest energy electrons occupy antibonding orbitals.
E) All of these are false.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is diamagnetic?
A) F2+
B) C2+
C) H2+
D) N2
E) N2+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Tetracyanoethylene has the skeleton shown here: 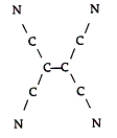 From its Lewis structure, determine the following.
-How many of the atoms are sp2 hybridized?
From its Lewis structure, determine the following.
-How many of the atoms are sp2 hybridized?
A) 2
B) 4
C) 10
D) 8
E) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the structure of glycine, the simplest amino acid: 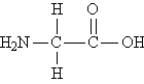 Indicate the hybridizations at each N and C atom in the molecule (in sequence from left to right) .
Indicate the hybridizations at each N and C atom in the molecule (in sequence from left to right) .
A) sp3 sp3 sp2
B) sp2 sp2 sp2
C) sp3 sp3 sp3
D) sp2 sp3 sp2
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Describing the bonding in C2H6 requires what carbon hybridization?
A) sp
B) sp3
C) dsp2
D) d2sp3
E) sp2
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Of the following homonuclear diatomic molecules, which is paramagnetic?
A) C2
B) B2
C) F2
D) N2
E) None of the above
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
What is the hybridization of I in ICl2+?
A) sp3
B) dsp3
C) sp2
D) sp
E) d2sp3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following molecule. (Lone pairs are not drawn in.) 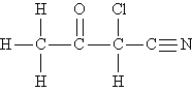 -What is the hybridization of the carbon atom that is bonded to chlorine?
-What is the hybridization of the carbon atom that is bonded to chlorine?
A) dsp3
B) sp3
C) sp
D) sp2
E) d2sp3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the I atom in the molecule IF5?
A) sp
B) sp3
C) dsp3
D) sp2
E) d2sp3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has the shortest N-O bond?
A) NO2-
B) N2
C) NO+
D) NO3-
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Specify the hybridization of the nitrogen atom in each of the following, in order. NO3- N2 NO2-
A) sp2, sp, sp2
B) sp3, sp, sp
C) sp2, sp, sp3
D) sp3, sp2, sp3
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Atoms that are sp3 hybridized form ____ pi bond(s) .
A) 1
B) 4
C) 2
D) 3
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the central atom in ICl5?
A) sp2
B) d2sp3
C) dsp3
D) sp
E) sp3
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Consider the following molecule. (Lone pairs are not drawn in.)  -What is the hybridization of the nitrogen atom?
-What is the hybridization of the nitrogen atom?
A) sp2
B) d2sp3
C) sp
D) sp3
E) dsp3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the molecular-orbital description of the NO- anion. Which of the following statements is false?
A) The bond energy in NO+ is greater than the bond energy in NO-.
B) The bond order in NO- is 2.
C) NO- is isoelectronic with CO.
D) NO- is paramagnetic.
E) All of these statements are false.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Oxygen is paramagnetic and has a bond order of two. Which of the following represents the ground electronic state for oxygen?
A) ![]()
B) ![]()
C) ![]()
D) A and C are equally probable
E) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The fact that O2 is paramagnetic can be explained by
A) hybridization of atomic orbitals in O2.
B) the Lewis structure of O2.
C) the molecular-orbital diagram for O2.
D) resonance.
E) a violation of the octet rule.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the phosphorus atom in PCl4+?
A) dsp2
B) sp2d
C) d2sp3
D) sp2
E) sp3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following Lewis structure. (Lone pairs are not drawn in.) 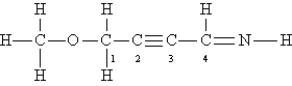 What are the hybridizations of the oxygen atom and of carbon atoms 1, 2, and 4, respectively (order: O C-1 C-2 C-4) ?
What are the hybridizations of the oxygen atom and of carbon atoms 1, 2, and 4, respectively (order: O C-1 C-2 C-4) ?
A) sp2 sp3 sp2 sp3
B) sp sp3 sp sp
C) sp3 sp3 sp sp2
D) sp sp2 sp sp2
E) sp sp3 sp2 sp
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 76
Related Exams