Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the compound shown below? 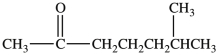
A) methyl hexyl ketone
B) 6, 6-dimethyl-2-hexanone
C) 6-methyl-2-heptanone
D) 2-methyl-6-heptanone
Correct Answer

verified
Correct Answer
verified
True/False
The IUPAC name of the molecule below is 3-ethoxy-3-propoxy-7-heptanal. 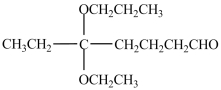
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of compound is shown below? 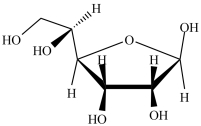
A) an epoxide
B) a cyclic hemiacetal
C) an aldehyde
D) a cyclic acetal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the condensed formula of the compound shown below? 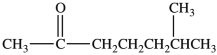
A) CH3CO(CH2) 3CH(CH3) 2
B) CH3CO(CH2) 3CHCH3CH3
C) CH3OC(CH2) 3CHCH3CH3
D) CH3OCCH2CH2CH2CH2CH(CH3) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What product results when cinnamaldehyde is oxidized? 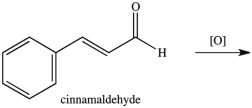
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of the compound below? 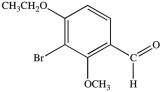
A) 3-bromo-4-ethyl-2-methylbenzaldehyde
B) 3-bromo-4-ethoxy-2-methoxybenzaldehyde
C) 1-bromo-2-ethoxy-6-methoxy-3-benzaldehyde
D) 3-bromo-4-ethoxy-2-methoxyphenal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the structure of 1-chloro-3-ethyl-2-heptanone?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Short Answer
When 2-methyl-3-pentanol is oxidized, the compound _____ is produced.
Correct Answer

verified
2-methyl-3...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What is the acetal formed in the reaction below? 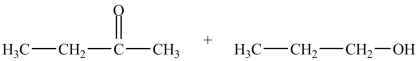
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
True/False
D-Glucose, shown below, will give a positive Tollens test. 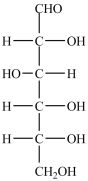
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of the compound below? 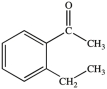
A) 2-ethylbenzenone
B) 2-ethylacetophenone
C) 2-ethylacetobenzone
D) 2-ethylphenone
Correct Answer

verified
Correct Answer
verified
True/False
Acetals are stable molecules, but they can be converted back to aldehydes and ketones by treatment with acid and water.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the compound shown below? 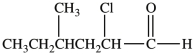
A) 5-chloro-3-methylhexanal
B) 2-chloro-4-methylhexanal
C) 5-chloro-3-methyl-6-hexanal
D) 2-chloro-4-methylhexanone
E) 3-chloro-5-methylhexanone
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What product results when cinnamaldehyde is oxidized? 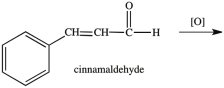
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the common name of the compound shown below? 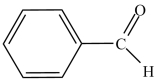
A) benzene aldehyde
B) benzaldehyde
C) acetaldehyde
D) phenone
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound is an example of a ketone?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of the above structures are ketones.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What reaction does the coenzyme NADH, in the presence of an enzyme, facilitate?
A) the reduction of a carbonyl group to form a carboxylic acid
B) the reduction of a carbonyl group to form an alcohol
C) the reduction of a carboxylic acid to form a carbonyl group
D) the oxidation of a carbonyl group to form an alcohol
E) the oxidation of a carbonyl group to form a carboxylic acid
Correct Answer

verified
Correct Answer
verified
True/False
The simplest ketone has a much higher boiling point than the simplest aldehyde.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the structure of 3-hydroxy-2-methylbenzaldehyde?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 103
Related Exams