A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
From the response list, select the correct number of constitutional isomers that exist for dichloroethanes
A) two
B) three
C) four
D) five
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a correct alkane structural formula?
A) CH3-CH2-CH3
B) CH3-CH3-CH2
C) CH2-CH2-CH2
D) CH2-CH3-CH2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following pairs of alkanes are the two members of the pair constitutional isomers?
A) hexane and 3-methylhexane
B) 2,4-dimethylhexane and 2,4-dimethylheptane
C) ethane and propane
D) 3-methylnonane and 3,4-dimethyloctane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Methane, the simplest alkane, is a major component of the atmospheres of Jupiter and Saturn but only a minor component of Earth's atmosphere. (2) Some solid-state alkanes find use as skin-softeners and skin-protectors. (3) HFO's are fluorinated hydrocarbons in which a carbon-carbon double bond is present.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the molecular formulas for cyclic and noncyclic alkanes with the same number of carbon atoms are compared, it is always found that the cycloalkane has
A) two more hydrogen atoms.
B) the same number of hydrogen atoms.
C) two less hydrogen atoms.
D) four less hydrogen atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many carbon atoms are present in the alkane whose IUPAC name is 2,2,4-trimethylpentane?
A) five
B) seven
C) eight
D) nine
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements concerning saturated hydrocarbons is incorrect?
A) Every carbon atom present has four bonds.
B) All bonds present are single bonds.
C) Every carbon atom present must be bonded to at least two hydrogen atoms.
D) More than one correct response.
E) No correct response.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alkanes has an IUPAC name that contains the word butane?
A) ![]()
B) ![]()
C) ![]()
D) more than one correct response
E) no correct response
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select from the response list a compound that has both primary and quaternary carbon atoms present.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) Natural gas and petroleum constitute the largest and most important sources of both alkanes and cycloalkanes. (2) The number of hydrogen atoms present in an alkane is always twice the number of carbon atoms plus two more. (3) Unbranched alkane boiling points decrease as carbon chain-length increases.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements concerning the boiling points of specific alkanes is correct?
A) Hexane has a higher boiling point than heptane.
B) Pentane has a higher boiling point than 2-methylpentane.
C) Butane has a higher boiling point than cyclobutane.
D) More than one correct response.
E) No correct response.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: (1) A minimum of four carbon atoms is required in a cycloalkane. (2) The molecules 2,4-dimethylpentane and 2,4-dimethylhexane are constitutional isomers. (3) As a result of rotation about single bonds, all alkane molecules, except methane, can exist in an infinite number of conformations.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The most important chemical use for alkanes relates to their chemical reaction with
A) hydrogen
B) oxygen
C) chlorine
D) bromine
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select from the response list a compound that has two secondary carbon atoms present.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements concerning cycloalkanes is correct?
A) Molecular formula always fits the general formula CnH2n+2.
B) All are hydrocarbons.
C) Each exists in two or more isomeric forms.
D) More than one correct response.
E) No correct response.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The representation 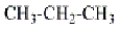 for the alkane propane is an example of
for the alkane propane is an example of
A) a molecular formula
B) a condensed structural formula
C) an expanded structural formula
D) a skeletal structural formula
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For which of the following cycloalkanes is cis-trans isomerism possible?
A) isopropylcyclopropane
B) propylcyclobutane
C) 1-methyl-1-propylcyclopropane
D) 1-ethyl-2-methylcyclodecane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following could not be the molecular formula for an alkane molecule?
A) C4H10
B) C5H14
C) CH4
D) C24H50
Correct Answer

verified
Correct Answer
verified
Multiple Choice
From the response list, select the correct number of constitutional isomers that exist for a four-carbon cycloalkane.
A) two
B) three
C) four
D) five
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 70
Related Exams