A) water
B) base in water
C) alcohol
D) carboxylic acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Carboxylic acids may be prepared by oxidation of
A) ketones
B) secondary alcohols
C) primary alcohols
D) tertiary alcohols
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following to answer the questions below: For each of the compound characterizations, select from the response list the number of constitutional isomers that exist. Responses may be used more than once or need not be used at all. -Chlorobenzoic acid
A) two
B) three
C) four
D) five
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds could not be oxidized to a carboxylic acid?
A) propanal
B) 2-methyl-1-propanol
C) 2-methyl-2-propanol
D) more than one correct response
E) no correct response
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is a constitutional isomer of ethyl ethanoate?
A) methyl methanoate
B) ethyl propanoate
C) butanoic acid
D) more than one correct response
E) no correct response
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following to answer the questions below: In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: -Statements: (1) Hydrolysis of a mixed acid anhydride produces two different carboxylic acids as products. (2) Short-chain unsubstituted monocarboxylic acids are strong acids, while their longer-chain counterparts are weak acids. (3) The parent alcohol for the ester methyl acetate is methyl alcohol.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following to answer the questions below: For each pair of carboxylic acids, select a correct characterization from the response list. Responses may be used more than once or need not be used at all. -Oxalic acid, Glutaric acid
A) Both are dicarboxylic acids.
B) Both are C3 acids.
C) Both are monohydroxy acids.
D) Both are unsubstituted monocarboxylic acids.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following to answer the questions below: For each of the compound characterizations, select from the response list the number of constitutional isomers that exist. Responses may be used more than once or need not be used at all. -Saturated four-carbon ester
A) two
B) three
C) four
D) five
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The IUPAC name for the compound 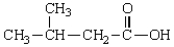 is
is
A) 2-methyl-4-butanoic acid
B) 2-methyl-4-pentanoic acid
C) 3-methylbutanoic acid
D) 3-methylpentanoic acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following to answer the questions below: For each of the compound characterizations, select from the response list the number of constitutional isomers that exist. Responses may be used more than once or need not be used at all. -Molecular formula of C3H6O2
A) two
B) three
C) four
D) five
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following to answer the questions below: Identify the missing reactant in each of the reactions using the response list. Responses may be used more than once or need not be used at all. -Acid + ? acid salt + H2O
A) water
B) base in water
C) alcohol
D) carboxylic acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements concerning carboxylate ions is correct?
A) They may be negatively or positively charged.
B) Carboxylate ions formed from dicarboxylic acids carry a -2 charge.
C) They contain more carbon atoms than their parent acids.
D) They are mostly positively charged.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The common names for the C2 mono- and dicarboxylic acids are, respectively,
A) formic acid and acetic acid
B) acetic acid and formic acid
C) oxalic acid and acetic acid
D) acetic acid and oxalic acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following types of inorganic esters would contain two oxygen-carbon single bonds?
A) diphosphate monoester
B) phosphate triester
C) diphosphate diester
D) more than one correct response
E) no correct response
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the general formula for a phosphate monoester?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The general structural difference between a carboxylic acid and an ester is the same as that between an
A) alcohol and an ether
B) aldehyde and a ketone
C) alkane and an alkene
D) more than one correct response
E) no correct response
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following carboxylic acids is paired with its correct IUPAC name?
A) ![]()
B) ![]()
C) ![]()
D) more than one correct response
E) no correct response
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following to answer the questions below: In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: -Statements: (1) "Pleasant odor" is a general characteristic of esters. (2) Citric acid is both a tricarboxylic acid and a ketoacid. (3) Esters and carboxylic acids with the same number of carbon atoms and the same degree of saturation are constitutional isomers.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following to answer the questions below: In each of the following multiple-choice questions, characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices: -Statements: (1) The boiling points of esters are much higher than those of corresponding carboxylic acids. (2) The compound benzoic acid is a heterocylic compound. (3) In linear form, the ester functional group can be represented as -COOR.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The common name for the C6 unbranched dicarboxylic acid is
A) pimelic acid
B) adipic acid
C) oxalic acid
D) succinic acid
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 64
Related Exams