A) ![]() M
M
B) ![]() M
M
C) ![]() M
M
D) ![]() M
M
E) ![]() M
M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of Ca(NO3) 2 must be added to 1.0 L of a 0.182 M KF solution to begin precipitation of CaF2? For CaF2,Ksp = 4.0 10-11.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The two salts AgX and AgY exhibit very similar solubilities in water.It is known that the salt AgX is much more soluble in acid than is AgY.What can be said about the relative strengths of the acids HX and HY?
A) Nothing.
B) HY is stronger than HX.
C) HX is stronger than HY.
D) The acids are weak acids and have equal values for Ka.
E) Both acids are strong.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The solubility of Mg(OH) 2 (Ksp = 8.9 10-12) in 1.0 L of a solution buffered (with large capacity) at pH 9.73 is:
A) ![]() moles
moles
B) ![]() moles
moles
C) ![]() moles
moles
D) ![]() moles
moles
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the concentration of the Cd2+(aq) ion in a 0.030 M Cd(NO3) 2 solution that is also 1.0 M NH3? At this temperature,Kf for Cd(NH3) 42+ = 1.0 107.
A) ![]() M
M
B) ![]() M
M
C) ![]() M
M
D) ![]() M
M
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the concentration of chromate ion,CrO42-,in a saturated solution of CaCrO4 (Ksp = 7.08 10-4) .
A) ![]() M
M
B) ![]() M
M
C) ![]() M
M
D) ![]() M
M
E) ![]() M
M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Ksp of Al(OH) 3 is 2 10-32.At what pH will a 0.5 M Al3+ solution begin to show precipitation of Al(OH) 3?
A) 3.5
B) 10.5
C) 1.0
D) 6.0
E) 3.1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molar solubility of AgCl (Ksp = 1.6 10-10) in 0.0035 M sodium chloride at 25°C is:
A) 0.0035
B) ![]()
C) ![]()
D) ![]()
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Ksp of AgI is 1.5 10-16.Calculate the solubility in mol/L of AgI in a 0.30 M NaI solution.
A) ![]()
B) 0.30
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The solubility of an unknown salt,MZ3, at 25°C is 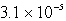 mol/L.What is the Ksp for MZ3 at 25°C?
mol/L.What is the Ksp for MZ3 at 25°C?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the limiting reagent in the formation of the lead chloride?
A) Pb2+
B) Cl-
C) (NO3) -
D) PbCl2
E) Pb(NO3) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following salts shows the lowest solubility in water? (Ksp values: Ag2S = 1.6 10-49;Bi2S3 = 1.0 10-72;HgS = 1.6 10-54;Mg(OH) 2 = 8.9 10-12;MnS = 2.3 10-13)
A) Bi2S3
B) Ag2S
C) MnS
D) HgS
E) Mg(OH) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Silver chromate,Ag2CrO4,has a Ksp of 8.96 10-12.Calculate the solubility in mol/L of silver chromate.
A) ![]() M
M
B) ![]() M
M
C) ![]() M
M
D) ![]() M
M
E) ![]() M
M
Correct Answer

verified
Correct Answer
verified
Showing 81 - 93 of 93
Related Exams