A) I
B) II,V
C) I,III,IV
D) I,II,III,IV
E) I,II,III,V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement is true?
A) All real processes are irreversible.
B) A thermodynamically reversible process takes place infinitely fast.
C) In a reversible process,the state functions of the system are always much greater than those of the surroundings.
D) There is always more heat given off to the surroundings in a reversible process than in an unharnessed one.
E) All statements (A-D) are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The value of H for this dissociation reaction is:
A) -137.3 kJ
B) 137.3 kJ
C) 1.986 kJ
D) -1.986 kJ
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction 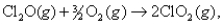 H° = 126.4 kJ/mol and S° = -74.9 J/K mol. At 361°C,what is G ?
H° = 126.4 kJ/mol and S° = -74.9 J/K mol. At 361°C,what is G ?
A) 153.4 kJ/mol
B) 47.6 kJ/mol
C) 173.9 kJ/mol
D) 78.9 kJ/mol
E) 155.0 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the process CHCl3(s) CHCl3(l) , H° = 9.19 kJ/mol and S° = 43.9 J/mol/K.What is the melting point of chloroform?
A) -64 °C
B) 209 °C
C) 130 °C
D) 64 °C
E) -130 °C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Substance X has a heat of vaporization of 58.4 kJ/mol at its normal boiling point (423°C) .For the process X(l) X(g) at 1 atm and 423°C calculate the value of Ssurr.
A) 0
B) 83.9 J/K mol
C) 138 J/K mol
D) -83.9 J/K mol
E) -138 J/K mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the dissociation reaction of the acid HF: HF(aq)  H+(aq) + F-(aq)
S is observed to be negative.The best explanation is:
H+(aq) + F-(aq)
S is observed to be negative.The best explanation is:
A) This is the expected result since each HF molecule produces two ions when it dissociates.
B) Hydration of the ions produces the negative value of S.
C) The reaction is expected to be exothermic and thus S should be negative.
D) The reaction is expected to be endothermic and thus S should be negative.
E) None of these can explain the negative value of S.
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
A 100-mL sample of water is placed in a coffee cup calorimeter.When 1.0 g of an ionic solid is added,the temperature decreases from 21.5°C to 20.8°C as the solid dissolves.For the dissolving of the solid
A) ( H < 0)
B) ( Suniv > 0)
C) ( Ssys< 0)
D) ( Ssurr > 0)
E) none of these
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
The third law of thermodynamics states:
A) The entropy of the universe is increasing.
B) The entropy of the universe is constant.
C) The entropy is zero at 0 K for a perfect crystal.
D) The absolute entropy of a substance decreases with increasing temperature.
E) The entropy of the universe equals the sum of the entropy of system and surroundings.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When ignited,solid ammonium dichromate decomposes in a fiery display.This is the reaction for a "volcano" demonstration.The decomposition produces nitrogen gas,water vapor,and chromium(III) oxide.The temperature is constant at 25°C.
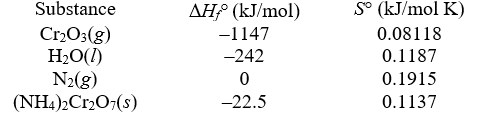 -Determine G° (in kJ/mol) .
-Determine G° (in kJ/mol) .
A) -191.4
B) -2281.4
C) -38.9
D) 1903.6
E) -1555.4
Correct Answer

verified
Correct Answer
verified
True/False
H° is zero for a chemical reaction at constant temperature.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The acid dissociation constant for a weak acid HX at 25°C is 4.3 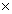 10-8.Calculate the free energy of formation for X-(aq) at 25°C.The standard free energies of HX(aq) and H+(aq) at 25°C are -208.3 kJ/mol and 0,respectively.
10-8.Calculate the free energy of formation for X-(aq) at 25°C.The standard free energies of HX(aq) and H+(aq) at 25°C are -208.3 kJ/mol and 0,respectively.
A) -205 kJ/mol
B) 250 kJ/mol
C) 0
D) -166 kJ/mol
E) -250 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the change in entropy of the surroundings for a process at 431 K and constant pressure is -326 J/K,what is the heat flow absorbed by for the system?
A) 326 kJ
B) 1.32 kJ
C) -141 kJ
D) 105 kJ
E) 141 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following reaction,CO2(g) + 2H2O(g)  CH4(g) + 2O2(g) , H° = 803 kJ which of the following will increase K?
CH4(g) + 2O2(g) , H° = 803 kJ which of the following will increase K?
A) decrease number of moles of methane
B) increase volume of system
C) increase the temperature of system
D) all of the above
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given that Hvap is 53.3 kJ/mol,and the boiling point is 83.4°C,1 atm,if one mole of this substance is vaporized at 1 atm,calculate G.
A) -150 J
B) 150 J
C) 639 J
D) -639 J
E) 0 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following system at equilibrium at 25°C:
PCl3(g) + Cl2(g)  PCl5(g)
for which H° = -92.5kJ at 25°C.
-When the volume is decreased at constant temperature,the ratio of the partial pressure of PCl5 to the partial pressure of PCl3 will
PCl5(g)
for which H° = -92.5kJ at 25°C.
-When the volume is decreased at constant temperature,the ratio of the partial pressure of PCl5 to the partial pressure of PCl3 will
A) increase
B) decrease
C) stay the same
D) impossible to tell without more information
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Assume that the enthalpy of fusion of ice is 6020 J/mol and does not vary appreciably over the temperature range 270-290 K.If one mole of ice at 0°C is melted by heat supplied from surroundings at 276 K,what is the entropy change in the surroundings,in J/K?
A) 22.1
B) 21.8
C) 0.0
D) -21.8
E) -22.1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Substance X has a heat of vaporization of 64.4 kJ/mol at its normal boiling point (423°C) .For the process X(l) X(g) at 1 atm and 423°C calculate the value of G.
A) 0 J
B) 92.5 J
C) 152 J
D) -92.5 J
E) -152 J
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
For a certain process at 355 K, G = -11.8 kJ and H = -9.2 kJ.Therefore, S for the process is
A) 0 J/K mol
B) 7.3 J/K mol
C) -7.3 J/K mol
D) -25.9 J/K mol
E) 25.9 J/K mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following system at equilibrium at 25°C:
PCl3(g) + Cl2(g)  PCl5(g)
for which H° = -92.5kJ at 25°C.
-When some Cl2(g) is added at constant volume and temperature,the ratio of the partial pressure of PCl5 to the partial pressure of PCl3 will
PCl5(g)
for which H° = -92.5kJ at 25°C.
-When some Cl2(g) is added at constant volume and temperature,the ratio of the partial pressure of PCl5 to the partial pressure of PCl3 will
A) increase
B) decrease
C) stay the same
D) impossible to tell without more information
E) none of these
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 117
Related Exams