A) Hg
B) Mg
C) Cu
D) Cd
E) Zn
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Four identical 1.0-L flasks contain the gases He,Cl2,CH4,and NH3,each at 0°C and 1 atm pressure. -For which gas are the molecules diatomic?
A) He
B) Cl2
C) CH4
D) NH3
E) all gases the same
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of gas is in a 50.0-mL container at a pressure of 645 torr and a temperature of 25°C.The entire sample is heated to a temperature of 35°C and transferred to a new container whose volume is 98.7 mL.The pressure of the gas in the second container is about:
A) 457 torr
B) 316 torr
C) ![]() torr
torr
D) 65 torr
E) 338 torr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate of effusion of an unknown gas was measured and found to be 11.9 mL/min.Under identical conditions,the rate of effusion of pure oxygen (O2) gas is 14.0 mL/min.Based on this information,the identity of the unknown gas could be:
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You are holding four identical balloons each containing 10.0 g of a different gas.The balloon containing which gas is the largest balloon?
A) H2
B) He
C) Ne
D) O2
E) All have the same volume.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
It is found that 250.mL of a gas at STP has a mass of 0.700 g.What is the molar mass?
A) 62.7 g/mol
B) 2.80 g/mol
C) 15.9 g/mol
D) 11.2 g/mol
E) 128 g/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A physics experiment is conducted at a pressure of 14.4 kPa.What is this pressure in mmHg?
A) 18.9 mmHg
B) 1.92 mmHg
C) ![]() mmHg
mmHg
D) 108 mmHg
E) ![]() mmHg
mmHg
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Three 1.00-L flasks at 25°C and 725 torr contain the gases CH4 (flask A) ,CO2 (flask B) ,and C2H6 (flask C) . -In which single flask do the molecules have the greatest mass,the greatest average velocity,and the highest kinetic energy?
A) Flask A
B) Flask B
C) Flask C
D) All are the same.
E) No one flask has all these.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Four identical 1.0-L flasks contain the gases He,Cl2,CH4,and NH3,each at 0°C and 1 atm pressure. -For which gas do the molecules have the smallest average kinetic energy?
A) He
B) Cl2
C) CH4
D) NH3
E) all gases the same
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The standard temperature for gases is
A) 100°C
B) 0°C
C) 32°C
D) 212°F
E) 0°F
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A mixture is prepared from 15.0 L of ammonia and 15.0 L chlorine measured at the same conditions;these compounds react according to the following equation: 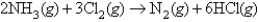 When the reaction is completed,what is the volume of each gas (NH3,Cl2,N2,and HCl,respectively) ? Assume the final volumes are measured under identical conditions.
When the reaction is completed,what is the volume of each gas (NH3,Cl2,N2,and HCl,respectively) ? Assume the final volumes are measured under identical conditions.
A) 0.00 L,5.00 L,7.50 L,45.0 L
B) 5.00 L,0.00 L,5.00 L,30.0 L
C) 0.00 L,0.00 L,7.50 L,45.0 L
D) 0.00 L,0.00 L,5.00 L,30.0 L
E) 0.00 L,10.0 L,15.0 L,90.0 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would represent the greatest pressure?
A) 0.680 atm
B) 517 mmHg
C) 11.4 psi
D) 62106 Pa
E) 14.1 in Hg
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Order the following in increasing rate of effusion: F2,Cl2,NO,NO2,CH4
A) Cl2 < NO2 < F2 < NO < CH4
B) Cl2 < F2 < NO2 < CH4 < NO
C) CH4 < NO2 < NO < F2 < Cl2
D) CH4 < NO < F2 < NO2 < Cl2
E) F2 < NO < Cl2 < NO2 < CH4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The bag is emptied and refilled,successively,with gases X and Y,this time at 1 atm pressure and a temperature 30°C higher.Assume that the volume of the bag is the same as before.Which one of the following statements is wrong?
A) The full bag contains fewer molecules of each gas than it did at 0.0°C.
B) The ratio of the density of gas Y to the density of gas X is the same as at 0.0°C.
C) The molar masses of the two gases are the same as they were at 0.0°C.
D) The mass of each gas filling the bag is now 303/273 times the mass held at 0.0°C.
E) The average velocity of the molecules of gas X at 30°C is higher than it was at 0.0°C.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not an assumption of the kinetic molecular theory for a gas?
A) Gases are made up of tiny particles in constant chaotic motion.
B) Gas particles are very small compared to the average distance between the particles.
C) Gas particles collide with the walls of their container in elastic collisions.
D) The average velocity of the gas particles is directly proportional to the absolute temperature.
E) All of the above are assumptions of the kinetic molecular theory.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A mixture of KCl and KClO3 weighing 1.34 grams was heated;the dry O2 generated occupied 143 mL at STP.What percent of the original mixture was KClO3,which decomposes as follows: 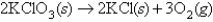
A) 38.9%
B) 58.4%
C) 87.6%
D) 10.7%
E) 23.7%
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All the following are postulates of the kinetic-molecular theory of gases except:
A) The collisions between molecules are elastic.
B) The gas molecules are in constant motion.
C) At a constant temperature,each molecule has the same kinetic energy.
D) The volumes of the molecules are negligible compared with the volume of the container.
E) The gas molecules are in rapid motion.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Four identical 1.0-L flasks contain the gases He,Cl2,CH4,and NH3,each at 0°C and 1 atm pressure. -For which gas are the collisions elastic?
A) He
B) Cl2
C) CH4
D) NH3
E) all gases the same
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas sample is held at constant pressure.The gas occupies 3.62 L of volume when the temperature is 21.6°C.Determine the temperature at which the volume of the gas is 3.42 L.
A) 312 K
B) 278 K
C) 20.4 K
D) 295 K
E) 552 K
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What would happen to the average kinetic energy of the molecules of a gas sample if the temperature of the sample increased from 20°C to 40°C?
A) It would double.
B) It would increase.
C) It would decrease.
D) It would become half its value.
E) Two of these.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 132
Related Exams