A) 130 L
B) 11 L
C) 17 L
D) 30.L
E) 5.6 L
Correct Answer

verified
Correct Answer
verified
Short Answer
A barometer is usually filled with ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At constant temperature,a sample of helium at 760.torr in a closed container was compressed from 5.00 L to 3.00 L.What was the new pressure exerted by the helium on its container?
A) 800.torr
B) 2280 torr
C) 15.0 torr
D) 3800 torr
E) 1270 torr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In Gay-Lussac's law,the pressure of a gas increases due to an increase in temperature because ________.
A) the molecules strike the walls of the container less often
B) the molecules strike the walls of the container more often
C) the molecules get bigger
D) there is a decrease in the volume of the container
E) there is an increase in the number of gas particles
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The force of gas particles against the walls of a container is called ________.
A) pressure
B) volume
C) temperature
D) quantity of gas
E) density
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A balloon is filled with helium gas.For the following question(s) ,select the letter of the balloon diagram that corresponds to the given change in conditions. 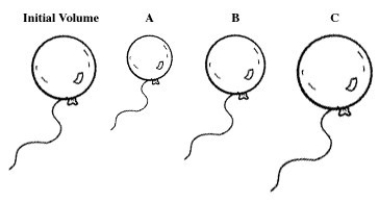 -The balloon is put into a chamber where the pressure is less than the atmospheric pressure.
-The balloon is put into a chamber where the pressure is less than the atmospheric pressure.
A) A
B) B
C) C
D) A and B
E) B and C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of argon at 300.°C and 50.0 atm pressure is cooled in the same container to a temperature of 0 °C What is the new pressure,if the volume and amount of gas do not change?
A) 105 atm
B) 45.5 atm
C) 54.9 atm
D) 23.8 atm
E) 42.7 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of nitrogen gas had a volume of 500.mL,a pressure in its closed container of 740 torr,and a temperature of 25 °C.What was the new volume of the gas when the temperature was changed to 50 °C and the new pressure was 760 torr,if the amount of gas does not change?
A) 530 mL
B) 450 mL
C) 970 mL
D) 240 mL
E) 400 mL
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At 570.mmHg and 25 °C,a gas sample has a volume of 2270 mL.What is the final pressure (in mmHg) at a volume of 1250 mL and a temperature of 175 °C,if the amount of gas does not change?
A) 1560 mmHg
B) 210 mmHg
C) 7000 mmHg
D) 690 mmHg
E) 470 mmHg
Correct Answer

verified
Correct Answer
verified
Short Answer
A gas has a pressure of 540.torr when the temperature is 127 °C.When the temperature changes to 27 °C,what is the new pressure,if there is no change in volume or amount of gas?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A balloon is filled with helium gas.For the following question(s) ,select the letter of the balloon diagram that corresponds to the given change in conditions. 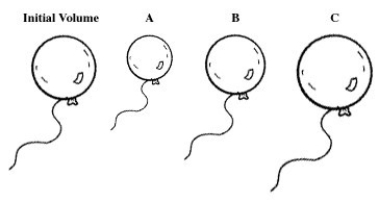 -The temperature is changed from 50 °C to -150 °C at constant pressure.
-The temperature is changed from 50 °C to -150 °C at constant pressure.
A) A
B) B
C) C
D) A and B
E) B and C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to Avogadro's law,________.
A) the volume of a gas is inversely related to the number of moles at constant temperature and pressure
B) the volume of a gas is inversely related to the number of moles at standard temperature and pressure
C) the volume of a gas depends only on the temperature and pressure
D) the volume of a gas depends only on the number of moles in the sample
E) the volume of a gas is directly related to the number of moles at constant temperature and pressure
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which measurement describes the pressure of a gas?
A) 315 K
B) 1.2 g/L
C) 2.5 L
D) 725 mmHg
E) 0.45 moles
Correct Answer

verified
Correct Answer
verified
True/False
The pressure exerted by a gas on its container is inversely related to its Kelvin temperature.
Correct Answer

verified
Correct Answer
verified
Short Answer
Nitrogen makes up about ________ percent of the atmosphere.
Correct Answer

verified
Correct Answer
verified
Short Answer
The total pressure of a mixture of CO2 and O2 is 780 mmHg.If the pressure of the CO2 is 560.torr,what is the pressure of O2?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At STP,what is the volume of 4.50 moles of nitrogen gas?
A) 167 L
B) 3420 L
C) 101 L
D) 60.7 L
E) 1230 L
Correct Answer

verified
Correct Answer
verified
Short Answer
A gas has a volume of 46.0 L when the temperature is 400.K.When the temperature changes to 500.K,what is the new volume,if there is no change in pressure or amount of gas?
Correct Answer

verified
Correct Answer
verified
True/False
The volume decreases as the temperature increases,if pressure and amount of gas do not change.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the kinetic molecular theory of gases,a gas can be compressed much more than a liquid or solid because ________.
A) a gas is composed of very small particles
B) the particles of a gas are very far apart
C) gas particles move rapidly
D) gas particles do not attract or repel one another
E) gas particles move faster when the temperature increases
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 76
Related Exams