A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of these choices.
Correct Answer

verified
Correct Answer
verified
Short Answer
According to Bronsted-Lowry theory,an acid is a substance that can ___.
Correct Answer

verified
donate a proton
Correct Answer
verified
Multiple Choice
Which of the reaction conditions would not afford the following transformation? 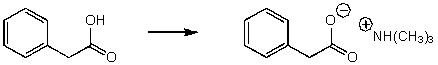
A) (CH3) 2NH
B) (CH3) 3N
C) (CH3) 2NLi
D) More than one of these choices.
E) None of these choices.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following acid/base reaction which statement is true taking S into consideration? 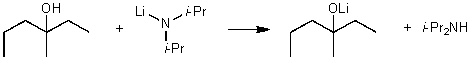
A) The reaction is an endothermic reaction and S is approximately zero.
B) The reaction is an endothermic reaction and S is negative.
C) The reaction is an exothermic reaction and S is negative.
D) The reaction is an exothermic reaction and S is approximately zero.
E) None of these choices.
Correct Answer

verified
Correct Answer
verified
True/False
The strongest acid that can exist in an aqueous solution is the hydronium ion H3O+.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following acid / base reaction which statement is true taking S into consideration? 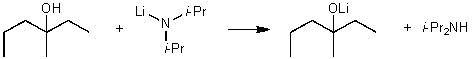
A) The reaction is an endothermic reaction and S is approximately zero.
B) The reaction is an endothermic reaction and S is negative.
C) The reaction is an exothermic reaction and S is negative.
D) The reaction is an exothermic reaction and S is approximately zero.
E) None of these choices.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the following reaction,which chemical species is acting like an electrophile? 
A) ![]()
B) ![]()
C) CH3O-
D) two of these choices
E) there is no electrophile in this reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following acid/base reaction which statement is true taking S into consideration? 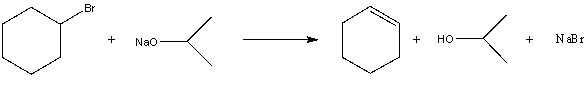
A) " G is negative and S is approximately zero"
B) " G is negative and S is negative"
C) " G is positive and S is positive"
D) " G is negative and S is positive"
E) "None of these choices."
Correct Answer

verified
Correct Answer
verified
Essay
Write an equation that shows the reaction between acetic acid (CH3COOH)and triethylamine (CH3CH2)3N.Draw all non-bonding lone electron pairs and show the electron flow with curved arrows.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the strongest acid?
A) CH3CH2OH
B) CH3CO2H
C) HC CH
D) CH2=CH2
E) CH3CH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is a true statement?
A) The stronger the acid,the larger is its pKa.
B) The conjugate base of a strong acid is a strong base.
C) Acid-base reactions always favor the formation of the stronger acid and the stronger base.
D) Strong acids can have negative pKa values.
E) Hydrogen need not be present in the molecular formula of a Bronsted-Lowry acid.
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
For the following acid / base reaction which statement is true taking H into consideration? 
A) The reaction is an exothermic reaction and H is approximately zero.
B) The reaction is an endothermic reaction and H is negative.
C) The reaction is an exothermic reaction and H is negative.
D) The reaction is an exothermic reaction and H is positive.
E) None of these choices.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 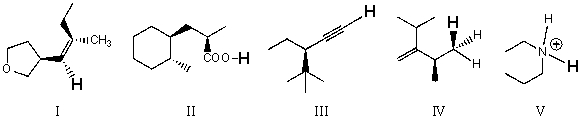
A) I > II > III > IV > V
B) II > V > III > I > IV
C) II > III > V > I > IV
D) III > I > V > II > IV
E) V > II > III > I > IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which reaction of these potential acids and bases does not occur to any appreciable degree due to an unfavorable equilibrium?
A) NaNH2 + CH3CH2CH2CH2CH2CH3
B) CH3CH2CO2Na + HI
C) CH3Li in hexane + ethyne
D) NaH + methanol
E) Two of these choices will not occur.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is an incorrect statement?
A) RSH compounds are stronger acids than ROH compounds.
B) PH3 is a weaker base than NH3.
C) NH2- is a stronger base than OH-.
D) OH- is a stronger base than OR-.
E) H- is a stronger base than OR-.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
As a consequence of the "leveling effect," the strongest acid which can exist in appreciable concentration in aqueous solution is:
A) H3O+
B) H2SO4
C) HClO4
D) HCl
E) HNO3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the acids below would have the strongest conjugate base?
A) CH3CH2OH pKa = 18
B) CH3CO2H pKa = 4.75
C) ClCH2CO2H pKa = 2.81
D) Cl2CHCO2H pKa = 1.29
E) Cl3CCO2H pKa = 0.66
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
What prediction can be made of the relative strengths of the conjugate bases of: H2S,HCl,SiH4,PH3?
A) PH2- > SiH3- > HS- > Cl-
B) SiH3- > PH2- > HS- > Cl-
C) Cl- > HS- > PH2- > SiH3-
D) HS- > Cl- > SiH3- > PH2-
E) Cl- > PH2- > SiH3- > HS-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 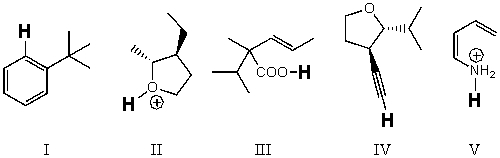
A) III > II > IV > V > I
B) II > III > V > IV > I
C) II > V > III > IV > I
D) V > III > II > IV > I
E) I > IV > II > III > V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What compounds are produced when NaSH is added to a mixture of water and methanol?
A) NaOH + NaOCH3 + H2S
B) H3O+ + Na2S + NaOCH3
C) H2SO4 + NaOCH3
D) H3O+ + NaOH + CH3SH
E) No reaction occurs
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 149
Related Exams