Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would afford a synthesis of the following compound? 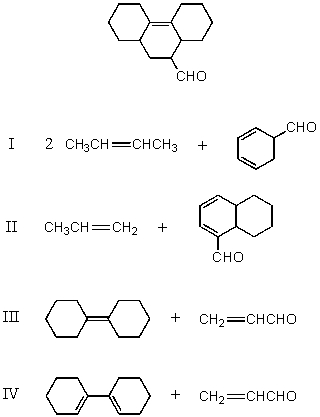
A) I
B) II
C) III
D) IV
E) None of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A thermodynamically-controlled reaction will yield predominantly:
A) the more/most stable product.
B) the product whose formation requires the smallest free energy of activation.
C) the product that can be formed in the fewest steps.
D) the product that is formed at the fastest rate.
E) the product which possesses the greatest potential energy.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these dienes can undergo the Diels-Alder reaction?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Essay
Draw arrows to indicate the electron flow for the following intramolecular Diels-Alder
cycloaddition reaction: 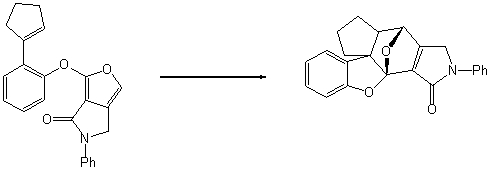
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrange these carbocations in order of expected increasing stability. 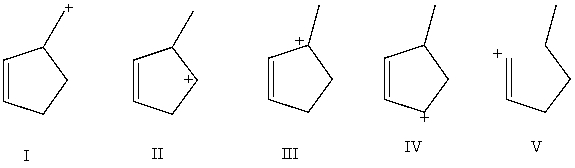
A) V < II < I < IV < III
B) V<I<II<IV<III
C) IV <I< II <V< III
D) IV < III < I < II < V
E) I < II < IV < III < V
Correct Answer

verified
Correct Answer
verified
Essay
What reagents would be needed to synthesize the following substance via the Diels-Alder reaction? Give stereochemical details,as relevant. 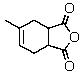
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which hydrogen atom(s) of 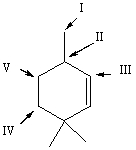 is/are most susceptible to abstraction by free radicals?
is/are most susceptible to abstraction by free radicals?
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Estimate the stabilization energy for 1,3-cyclohhexadiene using the heats of hydrogenation in Table 1. Table 1. Heats of Hydrogenation for Selected Compounds Compound Moles H2 H(kJ mol-1) Cyclohexene 1 -120 1,4-Cyclohexadiene 2 -240 1,3-Cyclohexadiene 2 -232 1,5-Hexadiene 2 -253
A) 13 kJ mol-1
B) 21 kJ mol-1
C) 8 kJ mol-1
D) 120 kJ mol-1
E) 112 kJ mol-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which alkene would you expect to have the smallest heat of hydrogenation?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which carbocation would be most stable?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following dienes is a cumulated diene?
A) CH2=CHCH2CH2CH2CH=CH2
B) CH2=CHCH=CHCH2CH2CH3
C) CH3CH=C=CHCH2CH2CH3
D) CH3CH=CHCH=CHCH2CH3
E) CH3CH2CH=CHCH2CH=CH2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What would be the product of the following reaction? 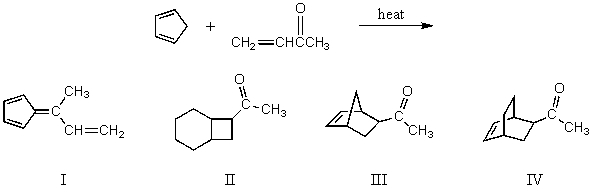
A) I
B) II
C) III
D) IV
E) All of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which diene would you expect to react most rapidly with maleic anhydride? 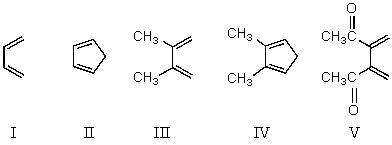
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What product(s) would you expect from the following substitution reaction of 14C-labeled propene? 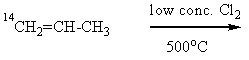
A) "14CH2=CH-CH2Cl alone"
B) "14CH2=CH-CH2Cl and CH2=CH-14CH2Cl,in equal amounts
C) CH2=CH-14CH2Cl alone"
D) "more 14CH2=CHCH2Cl,but a little CH2=CH-14CH2Cl"
E) "more CH2=CH-14CH2Cl,but a little 14CH2=CHCH2Cl"
Correct Answer

verified
Correct Answer
verified
Essay
Draw the structural formula for (2E,4Z,6E)-3,4,7,8-tetramethyl-2,4,6-nonatriene,clearly indicating stereochemical details.
Correct Answer

verified
Correct Answer
verified
Essay
Predict the product of the following Diels-Alder cycloaddition reaction: 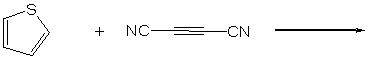
Correct Answer

verified
 Topic Die...
Topic Die...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following dienes would you expect to be the least stable?
A) CH3CH2CH=CHCH2CH=CHCH3
B) CH3CH=CHCH=CHCH2CH3
C) CH2=CHCH2CH2CH2CH=CH2
D) CH2=CHCH=CHCH2CH2CH3
E) CH3CH=C(CH3) CH=CHCH2CH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which carbocation would be most stable? 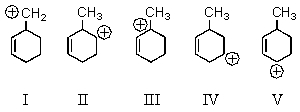
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which pair does not represent a pair of resonance structures? 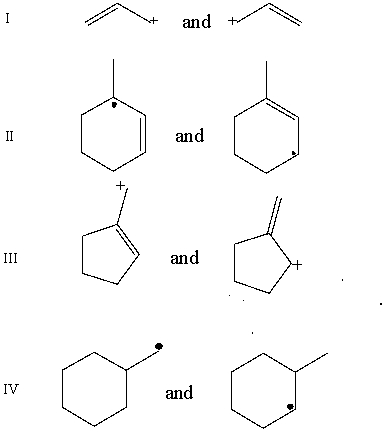
A) I
B) II
C) III
D) IV
E) All of these represent pairs of resonance structures.
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 166
Related Exams