A) 7
B) 8
C) 9
D) 10
E) 11
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following could be used to synthesize 1-bromopentane?
A) CH3CH2CH2CH=CH2 + HBr
B) CH3CH2CH2CH2CH2OH + PBr3
C) CH3CH2CH2CH2CH2OH + NaBr
D) CH3CH2CH2CH2CH2OH + Br2
E) CH3CH2CH2CH=CH2 + Br2
Correct Answer

verified
Correct Answer
verified
Essay
Draw all of the enantiomeric forms corresponding to the formula C5H12O.
Correct Answer

verified
Correct Answer
verified
Essay
Complete the following reaction sequence,giving structural details of all key intermediates: 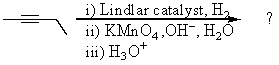
Correct Answer

verified
Correct Answer
verified
Short Answer
When an alcohol in which the OH is attached to a stereogenic carbon reacts with thionyl chloride (SOCl2)in the presence of a 3° amine,the resulting alkyl chloride is produced with _____________ of configuration.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the reaction of propyl alcohol with (CH3) 3SiCl in the presence of a tertiary amine?
A) CH3CH2CH2Si(CH3) 3
B) (CH3) 2CHSi(CH3) 3
C) CH3CH2CH2OSi(CH3) 3
D) (CH3) 2CHOSi(CH3) 3
E) (CH3CH2CH2) 3SiOH
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which of the following could be used to synthesize 2-bromobutane?
A) CH3CH2CH CH2 + Br2 (aq)
B) CH3CH2C CH3 + HBr
C) CH3CH2C CH + HBr
D) CH3CH2C CH + Br2
E) More than one of the above
Correct Answer

verified
Correct Answer
verified
Essay
Propose a mechanism for the following transformation: 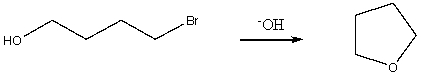
Correct Answer

verified
11ea9a02_1b4c_03e4_8bb6_c51e19f30e47_TB5901_00
Correct Answer
verified
Essay
Complete the following reaction sequence,giving structural details of all key intermediates: 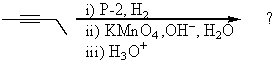
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction,  is probably:
is probably:
A) an SN1-type reaction involving the protonated alcohol as the substrate.
B) an SN2-type reaction involving the protonated alcohol as the substrate.
C) an E1-type reaction involving the protonated alcohol as the substrate.
D) an E2-type reaction involving the protonated alcohol as the substrate.
E) an epoxidation reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is NOT true of ethers?
A) Ethers are generally unreactive molecules toward reagents other than strong acids.
B) Ethers generally have lower boiling points than alcohols of a corresponding molecular weight.
C) Ethers generally have much lower water solubilities than alcohols with a corresponding molecular weight.
D) Ethers can generally be cleaved by heating them with strong acids.
E) Ethers form peroxides when allowed to stand in the presence of oxygen.
Correct Answer

verified
C
Correct Answer
verified
Essay
Complete the following reaction sequence,giving structural details of all key intermediates: 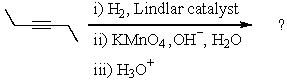
Correct Answer

verified
Correct Answer
verified
Essay
Propose a mechanism for the following transformation: 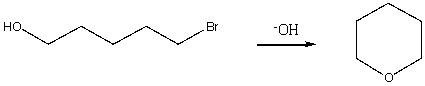
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the compounds listed below would you expect to have the highest boiling point? (They all have approximately the same molecular weight. )
A) CH3CH2CH2CH2CH3
B) CH3CH2CH2CH2OH
C) CH3CH2CH2OCH3
D) CH3CH2CH2Cl
E) CH3CH2OCH2CH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of secondary alcohols corresponding to the formula C5H12O,counting stereoisomers separately,is:
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If trans-2-butene is treated with meta-chloroperbenzoic acid what is the final product? 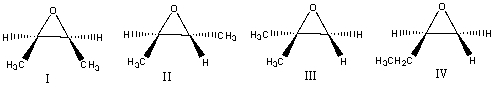
A) I
B) II
C) III
D) IV
E) None of the above
Correct Answer

verified
Correct Answer
verified
Essay
Complete the following reaction sequence,giving structural details of all key intermediates: 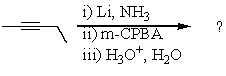
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the alcohols listed below would you expect to react most rapidly with HBr?
A) CH3CH2CH2CH2CH2CH2OH
B) (CH3CH2) 2CH2CH2OH
C) (CH3CH2) 2CHOHCH3
D) CH3CH2CH2CH2CH2OH
E) (CH3CH2) 2C(CH3) OH
Correct Answer

verified
Correct Answer
verified
Essay
Give the correct IUPAC name corresponding to the following structure: 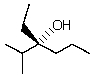
Correct Answer

verified
(R)-3-ethy...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which reagent(s) would transform propyl alcohol into propyl bromide?
A) Concd.HBr and heat
B) PBr3
C) NaBr/H2O and heat
D) More than one of these
E) All of these
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 172
Related Exams