A) s
B) p
C) sp
D) sp2
E) sp3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the atomic orbital.The lone pair electrons on the C atom are contained in: 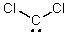
A) 2sp3
B) 2sp2
C) 2sp
D) 2s
E) 2p
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the geometry of the N in the following molecule? 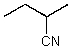
A) tetrahedral
B) trigonal pyramidal
C) linear
D) bent
E) trigonal planar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the N atom in the following molecule? 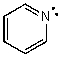
A) s
B) p
C) sp
D) sp2
E) sp3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the atomic orbitals in the C-O sigma bond in acetone. 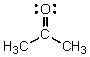
A) (2sp2,2sp2)
B) (2sp3,2sp3)
C) (2sp,2sp)
D) (2p,2p)
E) (2sp,1s)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Listed below are electron dot formulas for several simple molecules and ions.All valence electrons are shown;however,electrical charges have been omitted deliberately. 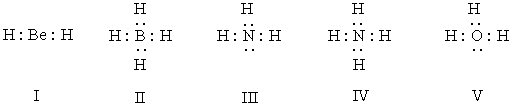 Which of the structures is negatively charged?
Which of the structures is negatively charged?
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Essay
Draw all the isomers of C4H10O,using bond-line formulas.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which structure(s) below does nitrogen have a formal charge of +1? 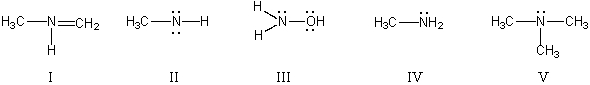
A) I
B) II and IV
C) III and V
D) I and V
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures is/are not a resonance form of the following species? 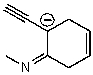
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of the above are correct resonance forms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the geometry of the N in the following molecule? 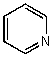
A) tetrahedral
B) trigonal pyramidal
C) linear
D) bent
E) trigonal planar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Considering Lewis structures,which of these compounds possesses a single unpaired electron?
A) N2
B) N2O
C) NO
D) N2O4
E) O2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the shortest of the carbon-carbon single bonds indicated by arrows in the following compounds?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following could not be a resonance structure of CH3NO2?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) Both C and D
Correct Answer

verified
Correct Answer
verified
Short Answer
Define an orbital.
Correct Answer

verified
A region of space wh...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Identify the atomic orbital the lone pair electrons on the O atom are contained in: 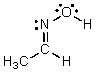
A) 2sp2
B) 2sp3
C) 2sp
D) 2s
E) 2p
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the atomic orbital.The lone pair electrons on the N atom are contained in: 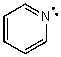
A) 2sp2
B) 2sp3
C) 2p
D) 2s
E) 2sp
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following would you expect the central atom to be sp3 hybridized (or approximately sp3 hybridized) ?
A) BH4-
B) NH4+
C) CCl4
D) CH3:-
E) All of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the C indicated with the arrow? 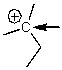
A) sp3
B) sp2
C) sp
D) s
E) p
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the atomic orbitals in the C-N sigma bond in the following oxime: 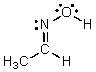
A) (2sp2,2sp2)
B) (2sp3,2sp3)
C) (2sp,2sp)
D) (2sp2,2sp3)
E) (2sp,1s)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these substances contain both covalent and ionic bonds?
A) NH4Cl
B) H2O2
C) CH4
D) HCN
E) H2S
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 134
Related Exams