A) the total energy of the universe is constant.
B) disorder in the universe is increasing with the passage of time.
C) it is theoretically impossible to convert work into heat with 100% efficiency.
D) the total energy in the universe is increasing with time.
E) the total energy in the universe is decreasing with time.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 2.00 kg piece of lead at 40.0°C is placed in a very large quantity of water at 10.0°C, and thermal equilibrium is eventually reached. Calculate the entropy change of the lead that occurs during this process. The specific heat of lead is 130 J/(kg∙K) .
A) -12.5 J/K
B) 86.0 J/K
C) -26.2 J/K
D) -86.0 J/K
E) -6.24 J/K
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A Carnot air conditioner operates between an indoor temperature of 20°C and an outdoor temperature of 39°C. How much energy does it need to remove 2000 J of heat from the interior of the house?
A) 105 J
B) 130 J
C) 780 J
D) 520 J
E) 340 J
Correct Answer

verified
Correct Answer
verified
Short Answer
A Carnot refrigerator takes heat from water at 0°C and rejects heat to a room at 12°C. Suppose that 92.0 grams of water at 0°C are converted to ice at 0°C by the refrigerator. Calculate the mechanical energy that must be supplied to the refrigerator. The heat of fusion of water is 3.34 × 105 J/kg.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An engine manufacturer makes the claim that the engine they have developed will, on each cycle, take 100 J of heat out of boiling water at 100°C, do mechanical work of 80 J, and exhaust 20 J of heat at 10°C. What, if anything, is wrong with this claim?
A) The heat exhausted must always be greater than the work done according to the second law of thermodynamics.
B) This engine violates the first law of thermodynamics because 100 J + 20 J ≠ 80 J.
C) An engine would operate by taking in heat at the lower temperature and exhausting heat at the higher temperature.
D) The efficiency of this engine is greater than the ideal Carnot cycle efficiency.
E) There is nothing wrong with this claim because 100 J = 20 J + 80 J.
Correct Answer

verified
Correct Answer
verified
True/False
According to the second law of thermodynamics, the entropy of any system always increases.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A heat engine performs the reversible cycle abca with 9.0 moles of an ideal gas, as shown in the figure. Path ca is an adiabatic process. The temperatures at points a and b are 300 K and 500 K, respectively. The volume at point c is 0.20 m3. The adiabatic constant of the gas is 1.60. The thermal efficiency of this engine is closest to 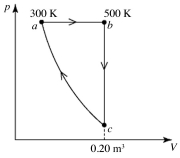
A) 0.070.
B) 0.10.
C) 0.13.
D) 0.16.
E) 0.19.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The graph in the figure shows a cycle for a heat engine for which QH =35 J. What is the thermal efficiency of this engine? 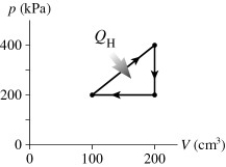
A) 29 %
B) 57 %
C) 14 %
D) 23 %
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A heat engine takes 2.0 moles of an ideal gas through the reversible cycle abca, on the pV diagram shown in the figure. The path bc is an isothermal process. The temperature at c is 820 K, and the volumes at a and c are 0.010 m3 and 0.16 m3, respectively. The molar heat capacity at constant volume, of the gas, is 37 J/mol·K, and the ideal gas constant is R = 8.314 J/(mol∙K) . The thermal efficiency of the engine is closest to 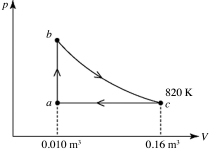
A) 0.26.
B) 0.026.
C) 0.33.
D) 0.40.
E) 0.53.
Correct Answer

verified
Correct Answer
verified
True/False
The entropy of an isolated system must be conserved, so it never changes.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 50 of 50
Related Exams