A) Alkynes undergo many addition reactions.
B) Alkynes are more polarizable than alkenes.
C) Both bonds of a C-C triple bond are weaker than a C-C bond.
D) The electrons in the bonds of alkynes are more tightly held than those of alkenes.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the addition of HBr to 1-butyne, the electrophile in the first step of the mechanism is:
A) The Csp-H1s bond of 1-butyne.
B) The C-C triple bond of 1-butyne.
C) The H atom in HBr.
D) The Br ion.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the IUPAC name for the following compound. 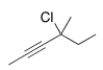
A) 4-Chloro-4-methyl-2-heptyne
B) 3-Chloro-3-methyl-4-heptyne
C) 3-Chloro-3-methyl-4-hexyne
D) 4-Chloro-4-methyl-2-hexyne
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
In the addition of HBr to 1-butyne, the nucleophile in the first step of the mechanism is:
A) The Csp-H1s bond of 1-butyne.
B) The C-C triple bond of 1-butyne.
C) The H atom in HBr.
D) The Br ion.
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which of the following molecular formulas is consistent with the formula for a molecule containing one alkyne and no other degrees of unsaturation?
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represents an internal alkyne? 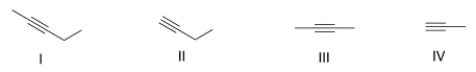
A) Only I and II
B) Only I and III
C) Only II and IV
D) Only II and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many degrees of unsaturation are introduced by a triple bond?
A) 0
B) 1
C) 2
D) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which method would work the best in accomplishing the following transformation? ![Which method would work the best in accomplishing the following transformation? A) [1] HBr; [2] 2eq. NaNH<sub>2</sub> B) [1] Br<sub>2</sub>; [2] 2eq. NaNH<sub>2</sub> C) [1] Br<sub>2</sub>, H<sub>2</sub>O; [2] NaNH<sub>2</sub> D) [1]BH<sub>3</sub>, THF; [2] H<sub>2</sub>O<sub>2</sub>, NaOH; [3] NaNH<sub>2</sub>](https://d2lvgg3v3hfg70.cloudfront.net/TB5871/11ea9088_70eb_442c_aec7_0b099c60fabd_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00_TB5871_00.jpg)
A) [1] HBr; [2] 2eq. NaNH2
B) [1] Br2; [2] 2eq. NaNH2
C) [1] Br2, H2O; [2] NaNH2
D) [1]BH3, THF; [2] H2O2, NaOH; [3] NaNH2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the IUPAC name for the following compound. 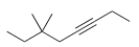
A) 6,6-Dimethyl-3-octyne
B) 3,3-Dimethyl-3-octyne
C) 3,3-Dimethy-5-octyne
D) 6-Ethyl-6-methyl-3-heptyne
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is a tautomer of 2-hexanone? 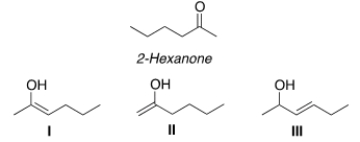
A) Only I and II
B) Only I and III
C) Only II and III
D) I, II, III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bases can deprotonate acetylene? You are given the pKa values of the conjugate acids in parentheses. I II III IV
A) Only I and II
B) Only I and III
C) Only II and III
D) Only II and IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major product of the following reaction? 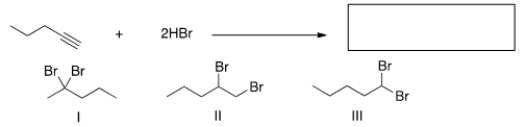
A) Only I
B) Only II
C) Only III
D) I, II, III
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which of the following alkynes is a symmetrical alkyne? 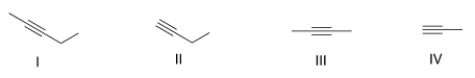
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represents a terminal alkyne? 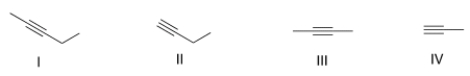
A) Only I and II
B) Only II and III
C) Only I and III
D) Only II and IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many bonds and bonds are present in a triple bond?
A) One bond and one bond.
B) One bond and two bonds.
C) Two bonds and one bond.
D) Two bonds and two bonds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the indicated hydrogen in the following compounds is the least acidic? 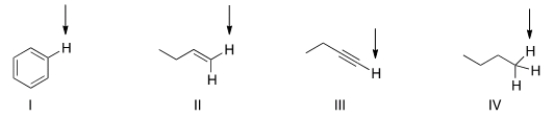
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product of the following reaction? 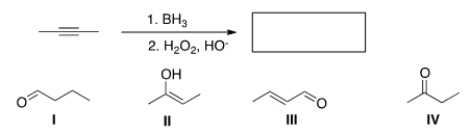
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product of the following reaction? 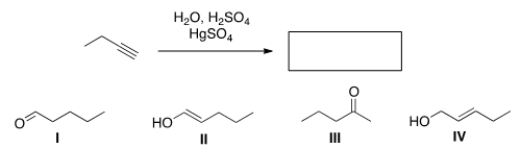
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following anions is the most basic? 
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about tautomers is true?
A) Tautomers differ in the position of a single bond and a hydrogen atom.
B) Tautomers differ in the position of a double bond and a carbon atom.
C) Tautomers differ in the position of a double bond and a hydrogen atom.
D) Tautomers differ in the position of a single bond and a carbon atom.
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 45
Related Exams