A) ![]()
=
![]()
B) ![]()
![]()
=
![]()
![]()
C) ![]()
=
![]()
D) ![]()
![]()
=
![]()
![]()
E) none of the above
G) C) and D)
Correct Answer

verified
Correct Answer
verified
True/False
If you had a five liter balloon of argon gas and a five liter balloon of xenon gas,and you removed 10 grams of gas from each balloon,the balloons would both shrink down to the same size.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the volume (in liters) of 1.00 mole of krypton gas that has a pressure of 1140 mm Hg and a temperature of 25.0°C? (R= 0.0821 L atm/ mol K)
A) 1.37
B) 16.3
C) 0.0215
D) 0.00180
E) none of the above
G) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Absolute zero refers to 0°C.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many liters of O2 (g) are needed to react completely with 56.0 L of CH4 (g) at STP to produce CO2 (g) and H2O (g) ? Given: CH4 (g) + 2O2 (g) → CO2 (g) + H2O (g)
A) 28.0 L
B) 56.0 L
C) 84.0 L
D) 112.L
E) none of the above
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the final pressure of a system (atm) that has the volume increased from 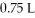 To
To 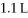 With an initial pressure of
With an initial pressure of 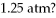
A) 1.1
B) 0.85
C) 1.8
D) 1.2
E) none of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A balloon originally had a volume of 0.439 L at 44°C and a pressure of 729 torr.To what temperature must the balloon be cooled to reduce its volume to 378 mL if the pressure remained constant?
A) 0°C
B) 38°C
C) 95°C
D) 273°C
E) none of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A barometer uses mercury because:
A) it is a convenient,safe,lightweight material.
B) the density of mercury is very large which allows the barometer to be short.
C) it is the traditional substance used,water could be as easily used.
D) it is the only liquid metal at room temperature.
E) All of the above are true.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
Vapor pressure of water increases with increasing temperature because the higher temperature causes more water molecules to evaporate.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of following statements are consistent with the Kinetic Molecular Theory?
A) Gases are compressible because the volume taken up by the gas is almost entirely open space.
B) Gases assume the shape and volume of their container because they are in constant,straight-line motion.
C) Gases have a low density because there is so much empty space between the particles.
D) Gas particles collide with each other and surfaces without losing any energy.
E) All of the above statements are consistent with the Kinetic Molecular Theory.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Pressure depends on how many gas particles are in a container.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Aerosol spray cans contain gas trapped in a fixed volume and cans of this type can explode if heated to high temperature.This illustrates that pressure and temperature are directly proportional.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The initial volume of a gas cylinder is 750.0 mL.If the pressure of a gas inside the cylinder changes from 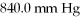 To
To 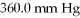 ,what is the final volume the gas occupies?
,what is the final volume the gas occupies?
A) 3.151 L
B) 630.0 mL
C) 1.750 L
D) 321.4 mL
E) none of the above
G) A) and E)
Correct Answer

verified
Correct Answer
verified
True/False
Gases and liquids are compressible,but solids are not.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Gas density can be calculated by dividing the mass of gas by its volume.If you took a balloon of gas and then warmed the balloon in a sunny window,what can now be said about the density of the gas in the balloon?
A) The gas density will remain the same.
B) The gas density will increase.
C) The gas density will decrease.
D) The density of gases is independent of temperature.
E) none of the above
G) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
If some argon gas at 400 mm Hg pressure is forced into a gas cylinder that already contained only neon gas at 400 mm Hg pressure,the total pressure in the cylinder would now be 800 mm Hg.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the equivalent pressure of 760 torr in units of mm Hg?
A) 760
B) 1
C) 14.7
D) 29.92
E) none of the above
G) D) and E)
Correct Answer

verified
Correct Answer
verified
True/False
The volume of 9.00 grams of water vapor at STP is 11.2 L.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At STP,12.69 g of a noble gas occupies 14.09 L.What is the identity of the noble gas? (R= 0.0821 L atm/ mol K)
A) He
B) Ne
C) Ar
D) Kr
E) not enough information
G) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
The vapor pressure of water is independent of temperature.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 122
Related Exams