Correct Answer

verified
Correct Answer
verified
True/False
The volume of a gas and the number of particles is inversely proportional.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the final volume of a gas that initially occupies 2.50 L at 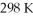 And is subsequently heated to
And is subsequently heated to 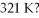
A) 2.69 L
B) 2.96 L
C) 2.23 L
D) 2.32 L
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When must temperature values in gas law calculations be expressed in kelvin units?
A) only for Charles's law
B) only for the Ideal Gas law
C) only for the Combined Gas law
D) never
E) always
Correct Answer

verified
Correct Answer
verified
True/False
A gas may not behave ideally under conditions of low pressure or high temperature.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT part of the Kinetic Molecular Theory?
A) Gas particles do not repel each other.
B) There is a large distance between gas particles as compared to their relative size.
C) The size of the actual gas particles is small compared to the volume of the whole gas.
D) The average energy of the particles is dependent on the molecular mass of the particle.
E) All of the above statements are part of the Kinetic Molecular Theory.
Correct Answer

verified
Correct Answer
verified
True/False
Pressure is calculated by: P =  .
.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If each of the following gas samples have the same temperature and pressure,which sample has the greatest volume?
A) 1 gram of O2
B) 1 gram of Ar
C) 1 gram of H2
D) all have the same volume
E) not enough information
Correct Answer

verified
Correct Answer
verified
True/False
Boyle's law states that as the volume of a gas increases so does the pressure.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the number of moles of a gas initially contained in a 2.10 L vessel is doubled,what is the final volume of the gas in liters? (Assume the pressure and temperature remain constant. )
A) 6.30
B) 1.05
C) 4.20
D) 8.40
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Water can be formed according to the equation: 2H2 (g) + O2 (g) → 2H2O (g) If 8.0 L of hydrogen is reacted at STP,exactly how many liters of oxygen at STP would be needed to allow complete reaction?
A) 4.0 L
B) 2.0 L
C) 1.0 L
D) 8.0 L
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A certain volume of gas was confined in a rigid container.If the pressure of the gas sample in the container was doubled,what happened to the temperature?
A) The Kelvin temperature decreased by one-half.
B) The Kelvin temperature doubled.
C) The Kelvin temperature increased four times.
D) The Kelvin temperature decreased one-third.
E) not enough information
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 325 mL sample of gas is initially at a pressure of 721 torr and a temperature of 32°C.If this gas is compressed to a volume of 286 mL and the pressure increases to 901 torr,what will be the new temperature of the gas (reported to three significant figures in °C) ?
A) 35.2°C
B) 335°C
C) 62.4°C
D) 215°C
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
If the number of gas particles is tripled,the volume will be 1/3 of the original given that temperature and pressure do not change.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the equivalent pressure of 1520 torr in units of atm?
A) 203,000
B) 380.
C) 2.00
D) 1520
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A rigid cylinder contains 2.00 liters of gas at a temperature of 25°C.If the pressure of this gas is changed from 0.500 atmospheres to 1.50 atmospheres,what will be the new temperature (in Kelvin,reported to three significant figures) of the gas? (The volume is constant. )
A) 99.3 K
B) 75.0 K
C) 8.33 K
D) 894 K
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about pressure is FALSE?
A) Pressure is caused by gas molecules colliding with surfaces.
B) The atmosphere has a pressure as the components of air collide with surfaces.
C) After creating a pressure difference,the atmospheric pressure can push liquid up a straw.
D) A deep well dug in the ground must have the pump located at the bottom of well in order to have the water come to the surface.
E) All of the above statements are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the third most abundant component of dry air?
A) carbon dioxide
B) oxygen
C) nitrogen
D) argon
E) smog
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What happens to the volume of a gas when you double the number of moles of gas while keeping the temperature and pressure constant?
A) The volume is halved.
B) The volume doubles.
C) The volume decreases,but more information is needed.
D) The volume increases,but more information is needed.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the equivalent pressure of 968 mm Hg in units of atm?
A) 1.27 atm
B) 0.785 atm
C) 968 atm
D) 1.30 atm
E) none of the above
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 122
Related Exams