A) Matter cannot be either created or destroyed in a chemical reaction.
B) The nucleus is a dense region of positive charge that always contains protons and neutrons.
C) All samples of a given compound have the same proportions of their constituent elements.
D) All atoms of a given element have a constant composition and are different than atoms of any other element.
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
The properties of a compound are an average of the properties of the individual elements.
Correct Answer

verified
Correct Answer
verified
True/False
The correct name for HNO3 is hydronitric acid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A certain oxyacid is derived from the oxyanion SO32-.The formula for the oxyacid is:
A) H2SO4.
B) HSO3.
C) H2SO3.
D) H3SO3.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the mass ratio of K to F in a compound is 2.06:1,how many grams of F are needed to react with 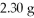 Of K?
Of K?
A) 0.877
B) 1.12
C) 0.112
D) 8.77
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of each type of atom are there in the formula Ca3(PO4) 2?
A) Ca = 3,P = 1,O = 4
B) Ca = 3,P = 2,O = 4
C) Ca = 3,P = 2,O = 8
D) Ca = 3,P = 1,O = 8
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Suppose that in an ionic compound,"M" represents a metal that could form more than one type of ion.In the formula MF2 ,the charge of the M ion would be:
A) +2
B) -1
C) +4
D) -2
E) +1
Correct Answer

verified
Correct Answer
verified
True/False
The name of KNO3 is potassium nitratide.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Suppose that in an ionic compound,"M" represents a metal that could form more than one type of ion.In the formula MP ,the charge of the M ion would be:
A) +1
B) -1
C) +2
D) -3
E) +3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of the molecular compound SF5?
A) sulfur hexafluoride
B) sulfur heptafluoride
C) monosulfur tetrafluoride
D) sulfur pentafluoride
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct formula for ammonium hydrogen sulfate?
A) NH4HSO4
B) (NH4) 2HSO4
C) (NH4) 2SO4
D) Am2HSO4
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
The correct name for H2SO3 is sulfurous acid.
Correct Answer

verified
Correct Answer
verified
True/False
Ionic compounds have a net charge of zero.
Correct Answer

verified
Correct Answer
verified
True/False
The ionic compound that forms between Mg and O is MgO.
Correct Answer

verified
Correct Answer
verified
True/False
The ratio in a chemical formula is a ratio of atoms,not a ratio of masses.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula for an ionic compound made of barium and nitrogen?
A) Ba3N2
B) Ba2N3
C) BaN
D) Ba2N4
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many carbon atoms are in the formula Al2(CO3) 3?
A) 3
B) 9
C) 1
D) 6
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
The basic unit of an ionic compound is called the formula unit.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula mass for diboron tetrachloride?
A) 127.98 amu
B) 198.89 amu
C) 234.34 amu
D) 163.43 amu
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of the compound whose formula is Na2O?
A) sodium monoxide
B) disodium oxide
C) disodium monoxide
D) sodium oxide
E) none of the above
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 110
Related Exams