A) noble gases
B) halogens
C) alkaline earth metals
D) alkali metals
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
Protons and electrons each have a mass of 1 amu.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Group 2A elements are also called:
A) noble gases.
B) halogens.
C) alkaline earth metals.
D) alkali metals.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A fictional element named Nivadium is found to have three naturally occurring isotopes with the natural abundances shown here: 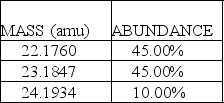 The calculated atomic mass of Nivadium is:
The calculated atomic mass of Nivadium is:
A) 7.61 amu
B) 22.83 amu
C) 23.18 amu
D) 69.55 amu
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the mass number of the hydrogen isotope that contains 2 neutrons?
A) 1
B) 2
C) 3
D) 4
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All of the following statements about different elements are true EXCEPT:
A) Barium is an alkaline earth metal.
B) Manganese is a transition metal.
C) Sulfur is considered a metalloid.
D) Krypton is one of the noble gases.
E) Iodine is a halogen.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following subatomic particles has a mass of 1.67 × 10-27 kg?
A) electrons only
B) protons only
C) neutrons only
D) protons and neutrons
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If an isotope of an element has 27 neutrons and a mass number of 52,how many electrons must it have?
A) 25
B) 27
C) 52
D) 79
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
All elemental symbols are comprised of a two-letter abbreviation.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement reflects the results of Rutherford's gold foil experiments?
A) Almost all of the alpha particles were deflected back in the direction from which they came.
B) Almost all of the alpha particles sputtered gold atoms off of the surface of the foil.
C) Almost all of the alpha particles were deflected while passing through the foil.
D) Almost all of the alpha particles passed directly through the foil.
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
In the modern periodic table,elements are listed in order of increasing atomic number rather than increasing relative mass.
Correct Answer

verified
Correct Answer
verified
True/False
The gold foil experiment proved that large regions of the atoms consisted of empty space.
Correct Answer

verified
Correct Answer
verified
True/False
An element is discovered that is a solid,has one valence electron,and readily forms a 1+ ion.This element would be correctly classified as a nonmetal.
Correct Answer

verified
Correct Answer
verified
True/False
The charges on electrons and neutrons cancel each other to give neutral atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom of a carbon-14 isotope would contain:
A) 6 protons,8 neutrons,and 6 electrons.
B) 8 protons,6 neutrons,and 8 electrons.
C) 6 protons,8 neutrons,and 8 electrons.
D) 14 protons,6 neutrons,and 6 electrons.
E) 20 protons,6 neutrons,and 20 electrons.
Correct Answer

verified
Correct Answer
verified
True/False
The atom is the fundamental building block of everything we hear,feel,see,and experience.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement below is NOT consistent with the nuclear theory of the atom as proposed by Rutherford?
A) Most of the atom's mass and all of its positive charge is contained in a small core called the nucleus.
B) Electrical charge is a fundamental property of protons and electrons in which like charges repel and opposite charges attract.
C) Most of the volume of the atom is empty space occupied by tiny,negatively charged electrons.
D) There are as many electrons outside the nucleus as there are protons inside the nucleus in a neutral atom.
E) All of the above statements are consistent.
Correct Answer

verified
Correct Answer
verified
True/False
Main-group elements tend to form ions that have the same number of total electrons as the nearest halogen.
Correct Answer

verified
Correct Answer
verified
True/False
The mass of a proton is exactly the same as the mass of a neutron.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Group 8A elements are also called:
A) noble gases.
B) halogens.
C) alkaline earth metals.
D) alkali metals.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 112
Related Exams