Correct Answer

verified
Correct Answer
verified
Essay
Draw the most stable conformer of trans-1-ethyl-4-methylcyclohexane.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has the greatest solubility in CH3CH2CH2CH3?
A) CH3OH
B) CH3O- Na+
C) CH3NH2
D) CH3OCH3
E) (CH3) 3CH
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the common name for the following structure? 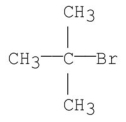
A) isobutyl bromide
B) t-Butyl bromide
C) neobutyl bromide
D) sec-Butyl bromide
E) isopropyl methyl bromide
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the compound with the highest bond angle.
A) the C-O-C bond in an ether
B) the C-N-C bond in a secondary amine
C) the C-N-C bond in a quaternary amine
D) the C-O-C bond in an alcohol
E) They are all equal.
Correct Answer

verified
Correct Answer
verified
Essay
What aspect of the fused ring systems present in cholesterol make it an ideal compound to lend rigidity to cell membranes?
Correct Answer

verified
The trans-fused ring systems p...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
Provide an acceptable name for the alkane shown below. 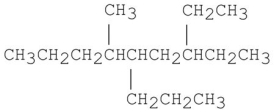
Correct Answer

verified
3-ethyl-6-...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
Provide an acceptable name for the alkane shown below. 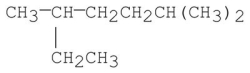
Correct Answer

verified
Correct Answer
verified
Essay
Which compound is more soluble in water? Briefly explain your choice. CH3OCH3 or CH3CH2OH
Correct Answer

verified
CH3CH2OH is more soluble in water since it...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
Provide an acceptable name for the alkane shown below. 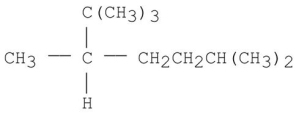
Correct Answer

verified
2,2,3,6-te...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Acetone has higher boiling point than propane because 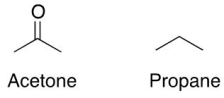
A) double bond in acetone is hard to break.
B) acetone has higher molecular weight.
C) acetone exhibits dipole-dipole interactions which are stronger than van der Waal's interactions in propane.
D) acetone exhibit hydrogen bonding which is stronger than van der Waal's interactions in propane.
E) acetone has higher surface area.
Correct Answer

verified
Correct Answer
verified
Essay
Provide an acceptable name for the compound shown below. 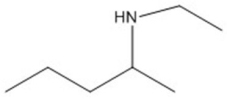
Correct Answer

verified
N-ethyl-2-...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Draw all ethers with molecular formula C4H10O.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Among the butane conformers,which occur at energy minima on a graph of potential energy versus dihedral angle?
A) gauche only
B) eclipsed and totally eclipsed
C) gauche and anti
D) eclipsed only
E) anti only
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has two equatorial alkyl substituents in its most stable conformation?
A) 1,1-dimethylcyclohexane
B) cis-1,2-dimethylcyclohexane
C) cis-1,3-diethylcyclohexane
D) cis-1,4-diethylcyclohexane
E) trans-1,3-diethylcyclohexane
Correct Answer

verified
Correct Answer
verified
Essay
There is something wrong with the following name.Write the structure and correct the name: 2-ethylpropane.
Correct Answer

verified
The correc...View Answer
Show Answer
Correct Answer
verified
View Answer
Showing 121 - 136 of 136
Related Exams