Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following stretching vibrations will appear at the highest frequency?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would you expect to have the longest λmax?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is consistent with the IR spectrum shown below? 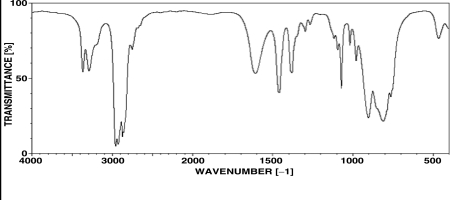
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would show a C  O stretch at the lowest frequency in its IR spectrum?
O stretch at the lowest frequency in its IR spectrum?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Short Answer
Label the axes on the IR spectrum below. 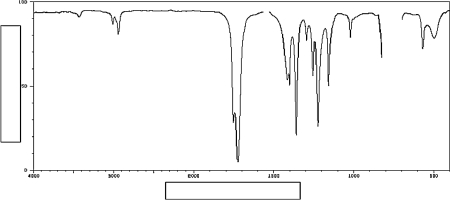
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of electron transition would create an absorption band at the longest wavelength in the UV-vis spectrum of the molecule below? 
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate energy difference between the HOMO and LUMO of a molecule that shows absorption bands at 286 nm,438 nm,and 472 nm in its UV-vis spectrum?
A) 4.54 × 10-19 J
B) 6.95 × 10-19 J
C) 7.24 × 10-19 J
D) 3.98 × 10-19 J
E) 4.21 × 10-19 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following wavelengths corresponds to the photon with the highest energy?
A) 385 nm
B) 425 nm
C) 318 nm
D) 538 nm
E) 647 nm
Correct Answer

verified
Correct Answer
verified
Essay
Briefly explain why acetone absorbs at a longer λmax than isobutylene. 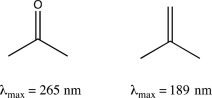
Correct Answer

verified
The HOMO of acetone is at a higher energ...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
An unknown compound has the molecular formula C3H7ON.Draw a structure of the unknown that is consistent with the IR spectrum below. 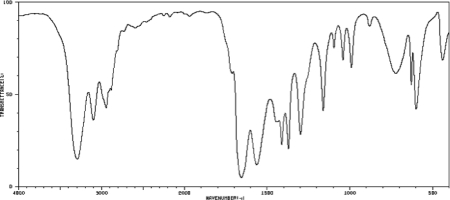
Correct Answer

verified
Correct Answer
verified
Essay
Which of the following compounds would be consistent with the IR spectrum shown below? Briefly explain your reasoning. 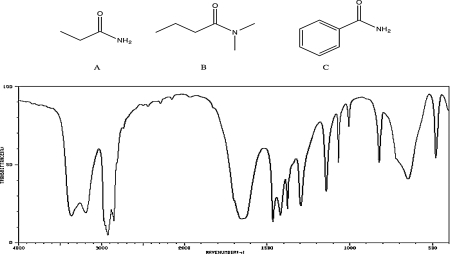
Correct Answer

verified
Compound A is the most consistent with t...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
What carbonyl-containing functional group is indicated by the IR spectrum below? 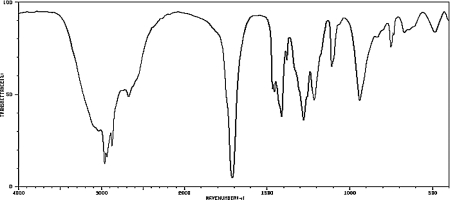
Correct Answer

verified
Correct Answer
verified
Short Answer
A compound whose molecular formula is C5H8O shows a strong absorption band at 1692 cm1.Draw a structure consistent with this information.
Correct Answer

verified
Correct Answer
verified
Essay
Describe how you could use IR spectroscopy to distinguish between the two isomers shown below. 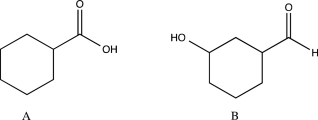
Correct Answer

verified
Compound A should exhibit a carboxylic a...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
What two functional groups are likely indicated by the peaks labeled A and B in the IR spectrum below? 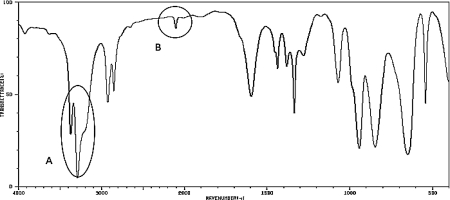
Correct Answer

verified
Peak A likely represents a pri...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Which of the two isomers shown below would exhibit a stronger C  C stretching absorption band? Explain your answer.
C stretching absorption band? Explain your answer. 
Correct Answer

verified
The isomer with the terminal alkene (B)w...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Rank the following in order of increasing C  O stretching frequency.
O stretching frequency. 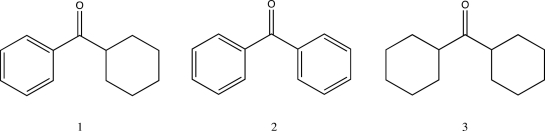
A) 2 < 1 < 3
B) 3 < 1 < 2
C) 1 < 2 < 3
D) 2 < 3 < 1
E) 3 < 2 < 1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds would have the lowest stretching vibrational frequency?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Short Answer
A compound has the molecular formula C7H6N2.Determine its structure based on the spectrum below. 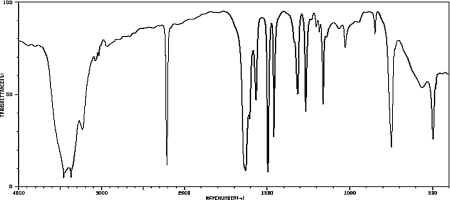
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 50
Related Exams