A) 1s2 2s2 2p0
B) 1s2 2s2 2p2
C) 1s2 2s22p3
D) 1s2 2s22p4
E) 1s2 2s2 2p6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
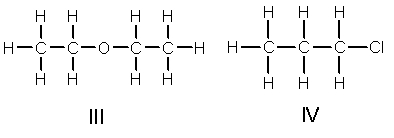
Rank the following compounds in decreasing order of boiling point. 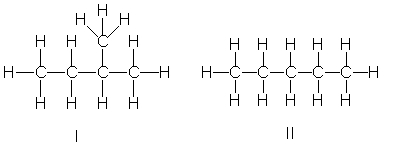
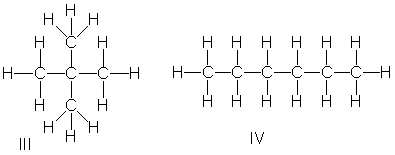
A) III > I > IV > II
B) II > I > IV > III
C) III > I > II > IV
D) IV > II > I > III
E) I > III > II > IV
Correct Answer

verified
Correct Answer
verified
Essay
Describe how soaps function as cleaning agents.
Correct Answer

verified
Soaps form clusters called micelles. The...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What is the molecular geometry at the central atom in CH2Br2?
A) trigonal planar
B) trigonal pyramidal
C) square planar
D) tetrahedral
E) None of these
Correct Answer

verified
Correct Answer
verified
Essay
Draw the Lewis structure for HCOOH and predict the hybridization state, molecular geometry and approximate bond angle around the central atom.
Correct Answer

verified
 _TB4454_00...
_TB4454_00...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Carbon is considered to be _______.
A) tetravalent
B) divalent
C) trivalent
D) monovalent
E) pentavalent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The C-C sigma bond in ethyne (H-C C-H) results from the overlap of which orbitals?
A) sp-sp
B) sp-sp3
C) sp2-sp2
D) sp-s
E) p-p
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct Lewis structure for hydrocyanic acid, HCN, including the formal charges, if any? 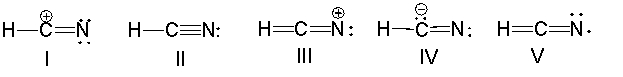
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molecular geometry of carbon tetrachloride, CCl4, is _________ .
A) tetrahedral
B) trigonal planar
C) trigonal pyramidal
D) square planar
E) linear
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is most soluble in water? 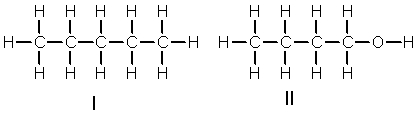
A) I
B) II
C) III
D) IV
E) II and III
Correct Answer

verified
Correct Answer
verified
Short Answer
Interaction of the following two atomic orbitals results in what kind of molecular orbital, in the orientation shown? 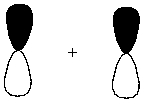
Correct Answer

verified
pi bonding...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following compounds has two lone pairs on the central atom?
A) CO2
B) SCl2
C) NF3
D) CS2
E) SO3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds does not have dipole moment?
A) HCl
B) NCl3
C) CO
D) BF3
E) All have dipole moment
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the compound with the longest carbon - nitrogen bond.
A) CH3CH2CH=NH
B) CH3CH2NH2
C) CH3CH2C≡N
D) The length of the carbon-nitrogen bonds are the same
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization state of the boron atom in the following compound? 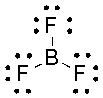
A) sp
B) sp2
C) sp3
D) sp3d
E) s2p
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures have a 1+ formal charge on the sulfur atom? 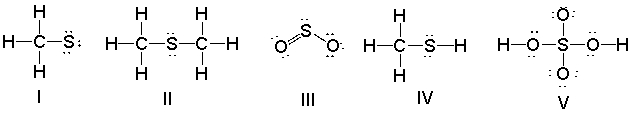
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Short Answer
According to molecular orbital theory, the destructive interference of two atomic orbitals results in a(n) _______.
Correct Answer

verified
antibondin...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Which of the following compounds has a dipole moment? Indicate the direction of the dipole moment. 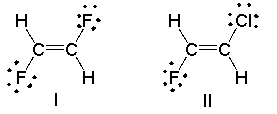
Correct Answer

verified
Compound I has no di...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following is the correct representation of the dipole for a P-Cl bond? 
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In quantum mechanics a node (nodal surface or plane) is ________ .
A) location where is negative
B) location where is positive
C) location where 2 is positive
D) location where 2 is negative
E) location where is zero
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 191
Related Exams