A) H2O
B) CO
C) H2
D) CO2
E) None
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule in the reaction below is the oxidizing agent? 2 C2H6(g) + 7 O2(g) 4 CO2(g) + 6 H2O(g)
A) C2H6
B) O2
C) H2O
D) CO2
E) None
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the oxidation number of each atom in sodium hydrogen carbonate,NaHCO3?
A) Na = +1, H = -1, C = +6,O = -2
B) Na = +1, H = +1, C = +4,O = -2
C) Na = +1, H = -1, C = +2,O = -2
D) Na = -1, H = +1, C = 0,O = -2
E) Na = 0, H = 0, C = 0,O = 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a weak electrolyte in aqueous solution?
A) Mg(OH) 2
B) NH3
C) NaOH
D) KOH
E) Ba(OH) 2
Correct Answer

verified
Correct Answer
verified
Short Answer
________ acid is produced in a larger quantity than any other chemical in the United States.This chemical is used in the production of fertilizers,pigments,alcohol,paper and detergents.
Correct Answer

verified
Correct Answer
verified
Short Answer
Ionic and molecular compounds that form ions in aqueous solution are called _____.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the balanced chemical equation for the complete combustion of benzoic acid,C6H5CO2H,to form carbon dioxide and water?
A) C6H5CO2H(s) 6 C(s) + CO2(g) + 3 H2(g)
B) C6H5CO2H(s) 7 CO2(g) + 3 H2O(g)
C) C6H5CO2H(s) + O2(g) CO2(g) + H2O(g)
D) C6H5CO2H(s) + 8 O2(g) 7 CO2(g) + 3 H2O(g)
E) 2 C6H5CO2H(s) + 15 O2(g) 14 CO2(g) + 6 H2O(g)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements concerning electrolytes and nonelectrolytes is/are true? 1) Some molecular substances are electrolytes. 2) All electrolytes are ionic substances. 3) Strong electrolytes partially ionize in solution.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 2 and 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What base results when Li2O reacts with water?
A) H3O+(aq)
B) LiH(aq)
C) O2-(aq)
D) LiOH(aq)
E) LiO-(aq)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is/are CORRECT? 1) Most ionic compounds containing nitrate ion are soluble in water. 2) Most ionic compounds containing sulfate ion are insoluble in water. 3) Most ionic compounds containing carbonate ion are soluble in water.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write a balanced chemical equation for the reaction of aqueous solutions of ammonium sulfate and sodium hydroxide.
A) (NH4) 2SO4(aq) + 2 NaOH(aq) 2 NH4OH(aq) + SO3(g) + Na2O(aq)
B) (NH3) 2SO4(aq) + NaOH(aq) 2 NH3(g) + NaOHSO4(aq)
C) (NH3) 2SO4(aq) + 2 NaOH(aq) 2 NH3(g) + Na2SO4(aq) + 2 OH-(aq)
D) (NH4) 2SO4(aq) + 2 NaOH(aq) 2 NH4+(g) + Na2SO4(aq) + 2 OH-(aq)
E) (NH4) 2SO4(aq) + 2 NaOH(aq) 2 NH3(g) + 2 H2O( ![]() ) + Na2SO4(aq)
) + Na2SO4(aq)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When solutions of barium chloride and lithium sulfate are mixed,the in the resulting precipitation reaction are
A) only SO42-.
B) both Li+ and Cl-.
C) only Cl-.
D) only Li+.
E) only Ba2+.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is in water?
A) Sc2O3
B) Cs3N
C) FeS
D) Hg2Br2
E) CdS
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A precipitate is expected when an aqueous solution of potassium iodide is added to an aqueous solution of
A) calcium nitrate.
B) barium hydroxide.
C) lead perchlorate.
D) iron(II) chloride.
E) sodium sulfate.
Correct Answer

verified
Correct Answer
verified
Short Answer
The net ionic equation for the reaction of barium chloride and sodium sulfate is shown below.Ba2+(aq)+ SO42-(aq) BaSO4(s) Chloride and sodium ions are referred to as ________ ions because they are not involved in the reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the oxidation number of each O in BaFeO4?
A) +6
B) +2
C) -2
D) +3
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which equation best represents the that occurs when an aqueous solution of barium bromide is mixed with an aqueous solution of sodium sulfate?
A) 2H+(aq) + 2Br-(aq) 2HBr(g)
B) Ba2+(aq) + SO42-(aq) BaSO4(s)
C) Ba2+(aq) + 2Br-(aq) + 2Na+(aq) + SO42-(aq) BaSO4(s) + 2NaBr(aq)
D) BaBr2(aq) + Na2SO4(aq) BaSO4(s) + 2NaBr(aq)
E) No net reaction occurs.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction of elemental chlorine with potassium iodide yields elemental iodine and potassium chloride.Write a balanced chemical equation for this reaction.
A) Cl2(g) + KI(s) I(s) + KCl2(s)
B) Cl2(g) + 2 KI(s) I2(s) + 2 KCl(s)
C) Cl2(g) + KI2(s) I2(s) + KCl2(s)
D) Cl(g) + KI(s) I(s) + KCl(s)
E) Cl2(g) + 2 K2I(s) I2(s) + 2 K2Cl(s)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following chemical equations show oxidation-reduction reactions? 1) Mg(s) + I2(aq) MgI2(s) 2) Pb(ClO4) 2(aq) + 2 KI(aq) PbI2(s) + 2 KClO4(aq) 3) Fe2O3(s) + 3 CO(g) 2 Fe(s) + 3 CO2(g)
A) 1 only
B) 2 only
C) 1 and 2
D) 1 and 3
E) 2 and 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The complete combustion of nonane,C9H20,yields carbon dioxide and water: 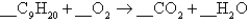 The coefficient of in the balanced equation is
The coefficient of in the balanced equation is
A) 15.
B) 12.
C) 16.
D) 13.
E) 14.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 85
Related Exams