A) ![]() C
C
B) ![]() He
He
C) ![]() B
B
D) ![]() Li
Li
E) ![]() B
B
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which particle changes the mass of the isotope the least?
A) alpha emission
B) proton capture
C) gamma particle
D) positron capture
E) electron capture
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the radiotracer used to for PET studies of the heart.
A) iron-59
B) phosphorus-32
C) thallium-201
D) iodine-131
E) fluorine-18
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify a gamma particle.
A) ![]() n
n
B) ![]() n
n
C) ![]() e
e
D) ![]() He
He
E) ![]() γ
γ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify a nuclide.
A) a particular radical
B) a particular cation
C) a particular atom
D) a particular isotope
E) a particular anion
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the identity of the daughter nuclide from the beta decay of 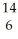 C.
C.
A) ![]() N
N
B) ![]() Be
Be
C) ![]() N
N
D) ![]() C
C
E) ![]() B
B
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write a nuclear equation to describe the spontaneous fission of 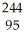 Am to form I-134 and Mo-107.Determine how many neutrons are produced in the reaction.
Am to form I-134 and Mo-107.Determine how many neutrons are produced in the reaction.
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the goal of the Manhattan project.
A) to build an hydrogen bomb
B) to build the first particle accelerator at Cornell
C) to build an atomic bomb
D) to build the first nuclear reactor to generate electricity for Manhattan
E) to set up the electrification of Manhattan using DC current
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction represents what nuclear process? 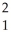 H +
H +  H →
H → 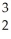 He +
He + 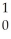 n
n
A) nuclear fusion
B) alpha emission
C) beta emission
D) neutron emission
E) neutron capture
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the identity of the daughter nuclide from the alpha decay of 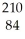 Po.
Po.
A) ![]() Rn
Rn
B) ![]() Pb
Pb
C) ![]() Ra
Ra
D) ![]() Hg
Hg
E) ![]() At
At
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the symptom that is NOT from radiation exposure.
A) measles
B) increased cancer risk
C) death
D) genetic effects
E) weaker immune systems
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the missing particle in the following nuclear equation: 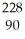 Th →
Th → 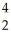 He + ?
He + ?
A) ![]() U
U
B) ![]() Ac
Ac
C) ![]() Ac
Ac
D) ![]() Ra
Ra
E) ![]() Ra
Ra
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following nuclides is most likely to undergo beta decay?
A) ![]() Pt
Pt
B) ![]() Pt
Pt
C) ![]() Pt
Pt
D) ![]() Pt
Pt
Correct Answer

verified
Correct Answer
verified
Essay
Describe what is meant by the term "valley of stability"?
Correct Answer

verified
If a plot of number of neutrons vs.numbe...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
What is the "mass defect"?
Correct Answer

verified
The mass of a nucleus is less than the s...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Write a nuclear equation for the alpha decay of 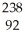 U.
U.
A) ![]() U →
U →![]() n +
n +![]() U
U
B) ![]() U →
U →![]() e +
e +![]() Np
Np
C) ![]() U →
U →![]() He +
He +![]() Th
Th
D) ![]() U →
U →![]() e +
e +![]() Pa
Pa
E) ![]() U →
U →![]() e +
e +![]() Pa
Pa
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Describe what changes occur during electron capture.
A) The mass number and atomic number decrease.
B) The mass number and atomic number increase.
C) The mass number is unchanged and the atomic number decreases.
D) The mass number decreases and the atomic number is unchanged.
E) The mass number and atomic number do not change.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is TRUE?
A) Alpha particles have the lowest penetrating power of any radioactivity.
B) Beta radiation has the lowest ionizing power of any radioactivity.
C) Beta emitters will do more damage than alpha emitters within the body.
D) Beta radiation has the highest ionizing power of any radioactivity.
E) None of the above is true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The nuclide As-76 has a half-life of 26.0 hours.If a sample of As-76 weighs 344 g,what mass of As-76 remains after 538 minutes?
A) 67.8 g
B) 271 g
C) 144 g
D) 437 g
E) 251 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the common radiotracers used in the diagnosis of medical problems.
A) fluorine-17
B) iodine-132
C) thallium-202
D) iron-58
E) iodine-131
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 135
Related Exams