A) KCl and Hg
B) NaI and C6H14
C) C3H8 and C2H5OH
D) F2 and PF3
E) NH3 and CH3OH
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the change in condition to go from a liquid to a gas.
A) increase heat or reduce pressure
B) increase heat or increase pressure
C) cool or reduce pressure
D) cool or increase pressure
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is expected to have the largest dispersion forces?
A) C H4
B) C9H20
C) F2
D) Si H4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the pair of substances that are most likely to form a homogeneous solution.
A) C6H14 and C10H20
B) LiCl and C5H12
C) N2O4 and NH4Cl
D) C6H14 and H2O
E) None of the pairs above will form a homogeneous solution.
Correct Answer

verified
Correct Answer
verified
Essay
Sketch the phase diagram of benzene.Make sure to label the axes and the different phases of benzene.Use the physical data provided below. melting point = 279 K boiling point = 353 K Tc = 562 K Pc = 48.4 atm Triple Point = 0.05 atm,279 K
Correct Answer

verified
Sketch should includ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
How much energy is required to vaporize 48.7 g of dichloromethane (CH2Cl2) at its boiling point,if its ΔHvap is 31.6 kJ/mol?
A) 31.2 kJ
B) 6.49 kJ
C) 55.1 kJ
D) 15.4 kJ
E) 18.1 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At atmospheric pressure,ice
A) freezes.
B) deposits.
C) sublimes.
D) melts.
E) boils.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The enthalpy change for converting 1.00 mol of ice at -25.0°C to water at 60.0°C is  The specific heats of ice,water,and steam are 2.09 J/gK,4.18 J/gK,and 1.84 J/gK,respectively.For H2O,ΔHfus = 6.01 kJ/mol,and ΔHvap = 40.67 kJ/mol.
The specific heats of ice,water,and steam are 2.09 J/gK,4.18 J/gK,and 1.84 J/gK,respectively.For H2O,ΔHfus = 6.01 kJ/mol,and ΔHvap = 40.67 kJ/mol.
A) 12.28
B) 6.31
C) 11.46
D) 5461
E) 9.58
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the substance with the highest vapor pressure at a given temperature.
A) SiS2
B) RbBr
C) CH3OCH2CH3
D) BH3
E) SbH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following substances would you predict to have the highest ΔHvap?
A) CH3Cl
B) HF
C) HOCH2CH2OH
D) CH3CH2OH
E) CH3CH2CH2CH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A paper clip floating on the top of water is an example of
A) capillary action.
B) viscosity.
C) hydrogen bonding.
D) surface tension.
E) dipole-dipole forces.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the term used to describe the ability of a liquid to flow against gravity up a narrow tube.
A) capillary action
B) viscosity
C) surface tension
D) density
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ethanol ( 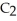
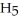 OH) melts at -114°C.The enthalpy of fusion is 5.02 kJ/mol.The specific heats of solid and liquid ethanol are 0.97 J/gK and 2.3 J/gK,respectively.How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -125°C to liquid ethanol at -80°C?
OH) melts at -114°C.The enthalpy of fusion is 5.02 kJ/mol.The specific heats of solid and liquid ethanol are 0.97 J/gK and 2.3 J/gK,respectively.How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -125°C to liquid ethanol at -80°C?
A) 207.3 kJ
B) -14.2 kJ
C) 4.95 kJ
D) 2224 kJ
E) 7.24 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds exhibits dipole-dipole forces as its strongest attraction between molecules?
A) H2
B) HBr
C) CO2
D) CH3NH2
E) Kr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the characteristics of a liquid.
A) indefinite shape and volume
B) indefinite shape, but definite volume
C) definite shape and volume
D) none of the above
E) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
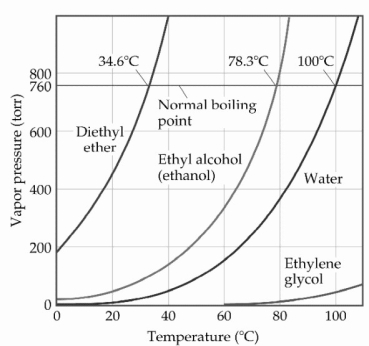 Based on the figure above,the boiling point of ethyl alcohol under an external pressure of 0.197 atm is ________°C.
Based on the figure above,the boiling point of ethyl alcohol under an external pressure of 0.197 atm is ________°C.
A) 80
B) 70
C) 60
D) 20
E) 40
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the substance with the highest viscosity.
A) diesel
B) water
C) maple syrup
D) motor oil
E) coffee
Correct Answer

verified
Correct Answer
verified
Multiple Choice
On a phase diagram,the fusion curve is between
A) a solid and a gas.
B) a solid and a liquid.
C) a liquid and a gas.
D) two solids.
E) two liquids.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The two strands in DNA are held together by
A) dispersion forces.
B) dipole-dipole forces.
C) hydrogen bonding.
D) ion-dipole forces.
E) ionic bonding.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is TRUE?
A) Vapor pressure increases with temperature.
B) Hydrogen bonds are stronger than covalent bonds.
C) Intermolecular forces hold the atoms in molecules together.
D) Dispersion forces are generally stronger than dipole-dipole forces.
E) None of the above is true.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 128
Related Exams