A) linear
B) trigonal pyramidal
C) seesaw
D) tetrahedral
E) bent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hybrid orbital set used by the central atom in NO2- is
A) sp.
B) sp2.
C) sp3.
D) sp3d.
E) sp3d2.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Describe a pi bond.
A) side by side overlap of p orbitals
B) end to end overlap of p orbitals
C) s orbital overlapping with the end of a p orbital
D) overlap of two s orbitals
E) p orbital overlapping with a d orbital
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule,that is sp3d hybridized and has a molecular geometry of linear,has ________ bonding groups and ________ lone pairs around its central atom.
A) 5, 1
B) 4, 2
C) 4, 1
D) 3, 2
E) 2, 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Soap works with water because
A) the polar head and the nonpolar tail attracts water.
B) the polar head attracts the grease and the nonpolar tail attracts water.
C) the polar head attracts water and the nonpolar tail attracts the grease.
D) the polar head and the nonpolar tail attracts the grease.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of ICl2⁻.
A) eg = tetrahedral, mg = trigonal bipyramidal
B) eg = tetrahedral, mg = trigonal pyramidal
C) eg = trigonal bipyramidal, mg = linear
D) eg = trigonal bipyramidal, mg = tetrahedral
E) eg = octahedral, mg = bent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the hybridization for the Br in BrO4⁻.
A) sp
B) sp3d2
C) sp3d
D) sp3
E) sp2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule with a T-shaped molecular geometry has a bond angle of
A) <120° for equatorial bonds and <90° for axial bonds.
B) 180°.
C) <90°.
D) 120° for equatorial bonds and 90° for axial bonds.
E) 120°.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of SeCl4?
A) seesaw
B) square planar
C) square pyramidal
D) tetrahedral
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the number of sigma bonds and pi bonds for benzene,C6H6.
A) 3 sigma bonds, 6 pi bonds
B) 6 sigma bonds, 6 pi bonds
C) 6 sigma bonds, 3 pi bonds
D) 12 sigma bonds, 3 pi bonds
E) 15 sigma bonds, 3 pi bonds
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of PF5.
A) eg = trigonal bipyramidal, mg = trigonal bipyramidal
B) eg = octahedral, mg = square pyramidal
C) eg = trigonal bipyramidal, mg = tetrahedral
D) eg = tetrahedral, mg = octahedral
E) eg = trigonal planar, mg = octahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The bond angle in H2Se is
A) 107°.
B) <104.5°.
C) 109.5°.
D) 190°.
E) 145°.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Describe a sigma bond.
A) side by side overlap of p orbitals
B) end to end overlap of p orbitals
C) s orbital overlapping with the side of a p orbital
D) overlap of two f orbitals
E) p orbital overlapping with a d orbital
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule containing a central atom with sp2 hybridization has a(n) ________ electron geometry.
A) linear
B) trigonal pyramidal
C) trigonal planar
D) tetrahedral
E) bent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) ,molecular geometry (mg) ,and polarity of SO2.
A) eg = tetrahedral, mg = tetrahedral, nonpolar
B) eg = trigonal planar, mg = bent, polar
C) eg = linear, mg = bent, nonpolar
D) eg = tetrahedral, mg = linear, nonpolar
E) eg = trigonal pyramidal, mg = trigonal pyramidal, polar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the molecular orbital diagram shown to determine which of the following is paramagnetic.
A) B22⁺
B) B22⁻
C) N22⁺
D) C22⁻
E) B2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the molecular orbital diagram shown to determine which of the following is most stable. 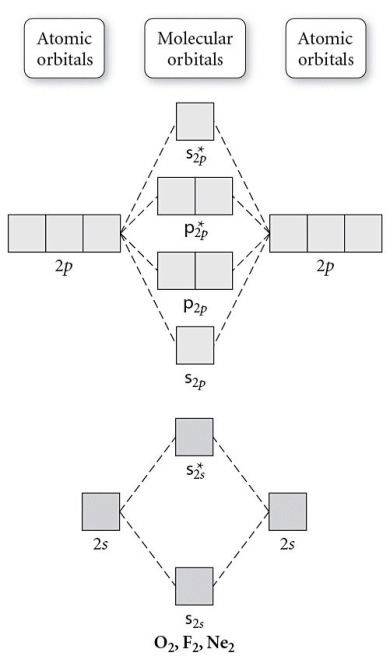
A) F2
B) F22⁺
C) Ne22⁺
D) O22⁺
E) F22⁻
Correct Answer

verified
Correct Answer
verified
Multiple Choice
List the number of sigma bonds and pi bonds in a triple bond.
A) 1 sigma, 1 pi
B) 2 sigma, 1 pi
C) 2 sigma, 2 pi
D) 1 sigma, 2 pi
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best describes IBrCl-? It has a molecular geometry that is
A) linear with no lone pairs on the I atom.
B) linear with lone pairs on the I atom.
C) nonlinear with no lone pairs on the I atom.
D) nonlinear with lone pairs on the I atom.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the hybridization for the S in SF6.
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 144
Related Exams