A) 1-cyclopentanone
B) 1-pentanone
C) 2-pentanone
D) 3-pentanone
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the consequence of the ability of aldehydes and ketones to form hydrogen bonds?
A) They are both highly colored when in the solid state.
B) They both have boiling points less than the comparable weight alcohol.
C) They prefer to hydrogen bond molecules of the same formula and will not dissolve well in water.
D) There is more than one correct response.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major difference between a cyclic hemiacetal and a cyclic acetal?
A) The cyclic hemiacetal is an alcohol,whereas the cyclic acetal is an ether.
B) The cyclic hemiacetal is an acid,and the cyclic acetal is a base.
C) The cyclic hemiacetal contains more carbons in the ring than the cyclic acetal.
D) All of the responses are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name for the following compound? 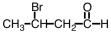
A) 3-bromobutanal
B) 2-bromobutanal
C) 3-bromobutanone
D) 2-bromobutanone
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following products is formed when hydrogen is reacted with 3-methyl-2-butanone?
A) primary alcohol
B) secondary alcohol
C) tertiary alcohol
D) acetal
Correct Answer

verified
Correct Answer
verified
True/False
It is not possible to produce either an aldehyde or ketone from a tertiary alcohol.
Correct Answer

verified
Correct Answer
verified
True/False
Aldehydes and ketones would always have a higher boiling point than the corresponding hydrocarbon with the same number of carbon atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name for compound shown below? 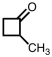
A) methylbutanone
B) 2-methylcyclobutanone
C) 1-methylcyclobutanal
D) 1-methylcyclobutanone
Correct Answer

verified
Correct Answer
verified
True/False
An aldehyde functional group must be on the end of a carbon chain.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is,in an injectable form,used for contraception?
A) norethynodrel
B) estrogen
C) progestin
D) testosterone
Correct Answer

verified
Correct Answer
verified
True/False
Acetals are generally more stable than hemiacetals.
Correct Answer

verified
Correct Answer
verified
True/False
The IUPAC name for formaldehyde is methanal.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the resultant compound class when an aldehyde is hydrogenated.
A) hemiketal
B) alcohol
C) carboxylic acid
D) no reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following pure compounds can exhibit hydrogen bonding with itself?
A) ![]()
B) ![]()
C) ![]()
D) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name for the compound shown below? 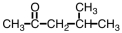
A) 2-methyl-2-pentanone
B) 2-methyl-4-pentanone
C) 4-methyl-2-pentanone
D) 2-methol-4-pentanol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an ingredient in some inhalants? It has a characteristic medicinal odor.
A) citral
B) biacetal
C) menthone
D) camphor
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The intermediate product of two moles of a primary alcohol and one mole of a ketone would be a(n) ____.
A) acetal
B) ketal
C) hemiacetal
D) hemiketal
Correct Answer

verified
Correct Answer
verified
True/False
Acetone is the common name for 2-butanone.
Correct Answer

verified
Correct Answer
verified
True/False
Generally,only those organic compounds containing oxygen or nitrogen can hydrogen bond.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds upon hydrolysis would yield CH3CH2OH and CH3CH2CHO?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 86
Related Exams