A) another ester + water.
B) alcohol + acid.
C) alcohol + water.
D) acid + water.
E) carboxylate salt + alcohol.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When an amide is hydrolyzed under basic conditions,the products are an
A) amine and a carboxylic acid.
B) amine and a carboxylate ion.
C) ammonium ion and a carboxylate ion.
D) ammonium ion and a carboxylic acid.
E) There is no reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the correct structure for N,N-diethyl benzamide?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Carboxylic acids generally taste
A) sour.
B) sweet.
C) bitter.
D) salty.
E) spicy.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which functional group contains a carbonyl group and an ether linkage bonded to the same carbon atom?
A) aldehyde
B) amide
C) carboxylic acid
D) ester
E) ketone
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When an alcohol reacts with phosphoric acid,the product is referred to as a
A) phosphate salt.
B) phosphate ester.
C) phosphate anion.
D) pyrophosphate.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When an amide is hydrolyzed under acidic conditions,the products are an
A) amine and a carboxylic acid.
B) amine and a carboxylate ion.
C) ammonium ion and a carboxylate ion.
D) ammonium ion and a carboxylic acid.
E) There is no reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -phosphate triester
A) a functional group consisting of a carbonyl carbon with a single bond to another oxygen;the remaining bonds are formed with R groups
B) a functional group consisting of an amine group bonded to a carbonyl carbon
C) the carbon atom bonded directly to the carbonyl carbon atom
D) a molecule that contains one phosphate ester linkage and two phosphoric anhydride linkages
E) the type of ester produced when phosphoric acid reacts with two molecules of alcohol
F) a functional group formed when two acid molecules give up one water molecule
G) the type of ester produced when phosphoric acid reacts with one molecule of alcohol
H) a molecule that contains one phosphate ester linkage and one phosphoric anhydride linkage
I) The common name of this compound is acetic acid.
J) The common name of this compound is formic acid.
K) a polymer produced by reacting diamines with diacids or diacyl chlorides
L) the transfer of a phosphoryl group from one molecule to another
M) the type of ester produced when phosphoric acid reacts with three molecules of alcohol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The most common reactions of carboxylic acids or their derivatives involve
A) addition across the double bond between carbon and oxygen.
B) replacement of the oxygen atom in the carbonyl group.
C) replacement of the group bonded to the carbonyl atom.
D) oxidation of the R group.
E) reduction of the R group.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the molecule shown? 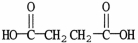
A) diethanoic acid
B) ethanedioc
C) dibutanoic acid
D) butanedioic acid
E) pentanedioic acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following conditions would favor the carboxylate ion form of a carboxylic acid?
A) low pH
B) high pH
C) both A and B
D) neither A nor B
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the common name of the molecule shown? 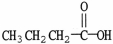
A) acetic acid
B) butyric acid
C) formic acid
D) lactic acid
E) oxalic acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following functional groups cannot hydrogen bond with itself?
A) carboxylic acids
B) esters
C) primary amines
D) alcohols
E) primary amides
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hydrolysis of the ester ethyl acetate produces
A) ethanol and acetic acid.
B) butanol.
C) butanal and ethanol.
D) ethanal and acetic acid.
E) butanoic acid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reactants needed to produce simple polyamides (nylons) are
A) diacids and dialcohols.
B) diacids and diamines.
C) diamines and dialcohols.
D) alkenes and catalysts.
E) diacids and phosphates.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name for the following compound? 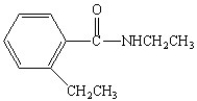
A) N,2-diethyl benzamide
B) diethyl benzamide
C) N,N-diethyl benzamide
D) ethyl phenyl amine
E) diethyl phenyl amine
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound is a carboxylic acid?
A) CH3COO-K+
B) CH3COOH
C) (CH3) 2CHOOCH3
D) (CH3CO) 2O
E) (CH3) 2O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -triphosphate
A) a functional group consisting of a carbonyl carbon with a single bond to another oxygen;the remaining bonds are formed with R groups
B) a functional group consisting of an amine group bonded to a carbonyl carbon
C) the carbon atom bonded directly to the carbonyl carbon atom
D) a molecule that contains one phosphate ester linkage and two phosphoric anhydride linkages
E) the type of ester produced when phosphoric acid reacts with two molecules of alcohol
F) a functional group formed when two acid molecules give up one water molecule
G) the type of ester produced when phosphoric acid reacts with one molecule of alcohol
H) a molecule that contains one phosphate ester linkage and one phosphoric anhydride linkage
I) The common name of this compound is acetic acid.
J) The common name of this compound is formic acid.
K) a polymer produced by reacting diamines with diacids or diacyl chlorides
L) the transfer of a phosphoryl group from one molecule to another
M) the type of ester produced when phosphoric acid reacts with three molecules of alcohol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The functional groups in the aspirin molecule shown are 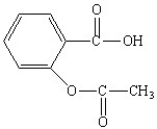
A) aromatic;carboxylic acid.
B) aromatic;ester.
C) carboxylic acid;ester.
D) aromatic;carboxylic acid;ester.
E) amide;aromatic;carboxylic acid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the molecule shown? 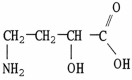
A) γ-amino-α-hydroxybutyric acid
B) 4-amino-2-hydroxybutanoic acid
C) α-amino-γ-hydroxybutyric acid
D) 1-amino-3-hydroxybutanoic acid
E) none of these
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 89
Related Exams