A) bent
B) linear
C) planar
D) pyramidal
E) tetrahedral
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
According to VSEPR theory,a molecule with three charge clouds including one lone pair would have a ________ shape.
A) bent
B) linear
C) tetrahedral
D) trigonal planar t
E) pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A triple bond involves the sharing of ________ pairs of electrons between the atoms
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer

verified
Correct Answer
verified
Essay
Explain how it is possible for CCl4 to have polar bonds but be a non-polar molecule.A diagram may be helpful in your answer,but it must be explained.
Correct Answer

verified
(This question could also be asked about...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Match the following. -sulfur hexafluoride
A) ICl
B) C ![]()
C) N ![]()
D) PC ![]()
E) ![]()
![]()
F) CO
G) S ![]()
H) S ![]()
I) O ![]()
J) Br ![]()
K) CC ![]()
L) I ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In forming covalent bonds where the octet rule is obeyed,sulfur usually forms ________ bonds and chlorine usually forms ________ bonds.
A) one;one
B) two;two
C) one;two
D) two;one
E) six;seven
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
The number of valence electrons in the acetic acid molecule (CH3CO2H) is
A) 0.
B) 8.
C) 16.
D) 24.
E) 32.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following structures is the correct structure for SOCl2?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -iodine monochloride
A) ICl
B) C ![]()
C) N ![]()
D) PC ![]()
E) ![]()
![]()
F) CO
G) S ![]()
H) S ![]()
I) O ![]()
J) Br ![]()
K) CC ![]()
L) I ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is likely to have ionic bonds between the atoms?
A) CH4
B) CaCl2
C) NBr3
D) SO2
E) All of these have covalent bonds.
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Consider the molecule SiCl4.The electronegativity values for Si and Cl are 1.8 and 3.0,respectively.Based on these values and on consideration of molecular geometry,the Si-Cl bond is ________ and the molecule is ________.
A) polar;polar
B) nonpolar;nonpolar
C) polar;nonpolar
D) nonpolar;polar
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -bromine pentafluoride
A) ICl
B) C ![]()
C) N ![]()
D) PC ![]()
E) ![]()
![]()
F) CO
G) S ![]()
H) S ![]()
I) O ![]()
J) Br ![]()
K) CC ![]()
L) I ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemical bond formed when two atoms share one pair of electrons is a ________ bond;it is best described as ________.
A) double;covalent
B) double;ionic
C) single;covalent
D) single;ionic
E) triple;covalent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which representation of a hydrogen molecule is not correct?
A) H=H
B) H2
C) H:H
D) H-H
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A bond where the electrons are shared unequally is called a(an) ________ bond.
A) polar covalent
B) coordinate covalent
C) nonpolar covalent
D) ionic
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which representation of a methane molecule is not correct? (A methane molecule is composed of one carbon atom and four hydrogen atoms. )
A) C ![]()
B) ![]()
C) ![]()
D) ![]()
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule that contains three identical polar bonds to the central atom will be
A) nonpolar if the geometry is planar triangular.
B) polar in all cases.
C) nonpolar in all cases.
D) impossible to tell the polarity.
E) either polar or nonpolar depending on the identity of the atoms bonded to the central atom.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element is most likely to make two covalent bonds in the formation of a molecular compound?
A) phosphorus
B) sulfur
C) bromine
D) nitrogen
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The total number of valence electrons in a molecule of SOF2 is
A) 26.
B) 24.
C) 18.
D) 20.
E) 22.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element is most likely to be "X" in the diatomic molecule shown? 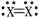
A) nitrogen
B) oxygen
C) fluorine
D) hydrogen
E) helium
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 78
Related Exams