A) 2.56
B) 62.5
C) 1.28 × 102
D) 1.53 × 103
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction A2 + B2 ⇌ 2 AB has an equilibrium constant Kc = 1.8.The following pictures represent reaction mixtures that contain A2 molecules (shaded) and B2 molecules (unshaded) ,and AB molecules.Which reaction mixture is at equilibrium? 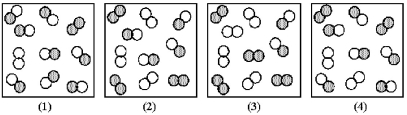
A) reaction mixture (1)
B) reaction mixture (2)
C) reaction mixture (3)
D) reaction mixture (4)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the equilibrium equation for the reaction: NH4NO3(s) ⇌ N2O(g) + 2 H2O(g) ?
A) Kp = [N2O]
B) Kp = [N2O][H2O]
C) Kp = [N2O][H2O]2
D) Kp = ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following will result in an increase in the amount of NH4Cl? NH4Cl (s) ⇌ NH3 (g) + HCl (g)
A) increasing the volume
B) decreasing the amount of HCl (g)
C) Iincreasing the amount of NH3 (g)
D) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At a certain temperature,Kc equals 1.4 × 102 for the reaction: 2 CO(g) + O2(g) ⇌ 2 CO2(g) . If a 2.50-L flask contains 0.400 mol of CO2 and 0.100 mol of O2 at equilibrium,how many moles of CO are also present in the flask?
A) 0.422 mol
B) 0.169 mol
C) 0.107 mol
D) 0.0114 mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Picture (1) represents an equilibrium mixture of solid CaCO3,solid CaO,and gaseous CO2,obtained as a result of the endothermic decomposition of CaCO3. 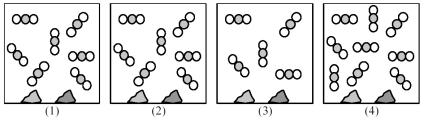 -Which picture (2) -(4) represents the equilibrium mixture when more solid CaCO3 is added?
-Which picture (2) -(4) represents the equilibrium mixture when more solid CaCO3 is added?
A) picture (2)
B) picture (3)
C) picture (4)
D) All of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Picture (1) represents an equilibrium mixture of solid CaCO3,solid CaO,and gaseous CO2,obtained as a result of the endothermic decomposition of CaCO3.  -Which picture (2) -(4) represents the equilibrium mixture when more solid CaO is added?
-Which picture (2) -(4) represents the equilibrium mixture when more solid CaO is added?
A) picture (2)
B) picture (3)
C) picture (4)
D) All of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At 25°C,a certain first order reaction has a rate constant equal to 1.00 × 10-3 s-1 and an equilibrium constant,Kc,equal to 4.18.What is the rate constant for the reverse reaction?
A) 2.39 × 10-4 s-1
B) 4.18 × 10-3 s-1
C) 2.39 × 102 s-1
D) 4.18 × 103 s-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Shown below is a concentration vs.time plot for the reaction A ⇌ 2B.For this reaction the value of the equilibrium constant is 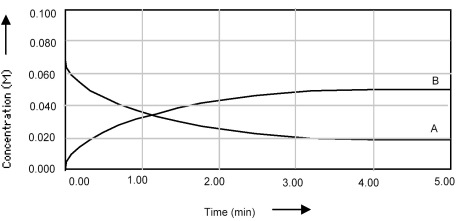
A) Kc < 1.
B) Kc = 0.
C) Kc = 1.
D) Kc > 1.
Correct Answer

verified
Correct Answer
verified
Short Answer
For the reaction 2 A + B2 ⇌ 2 AB,the rate of the forward reaction is 0.75 M/s and the rate of the reverse reaction is 0.25 M/s.The reaction is not at equilibrium.In order to attain equilibrium the reaction must proceed in the ________ (forward,reverse)direction in order to achieve equilibrium.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a homogeneous equilibrium of gases,which of the following changes in reaction conditions will not alter the equilibrium concentrations?
A) addition of an inert gas to the reaction mixture
B) addition of products
C) increasing the volumee
D) increasing the temperature
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Shown below is a concentration vs.time plot for the reaction A ⇌ B.For this reaction the value of the equilibrium constant is 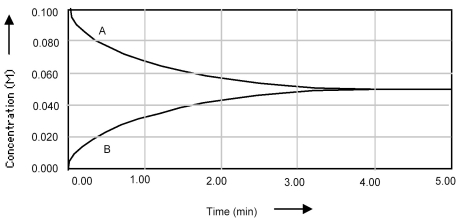
A) Kc < 1.
B) Kc = 0.
C) Kc = 1.
D) Kc > 1.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The solubility of 1:1 salts is measured by the equilibrium constant for the general reaction: MX(s) = Mn+(aq) + Xn-(aq) .Given the following salts and their equilibrium constants for the reaction above at 25°C,which salt is the least soluble?
A) MgCO3,Kc = 6.8 × 10-6
B) CaCO3,Kc = 5.0 × 10-9
C) SrCO3,Kc = 5.6 × 10-10
D) BaCO3,Kc = 2.6 × 10-9
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Picture (1) represents an equilibrium mixture of solid CaCO3,solid CaO,and gaseous CO2,obtained as a result of the endothermic decomposition of CaCO3.  -Which picture (2) -(4) represents the equilibrium mixture at a higher temperature?
-Which picture (2) -(4) represents the equilibrium mixture at a higher temperature?
A) picture (2)
B) picture (3)
C) picture (4)
D) None of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A crude type of disappearing ink is based on the following endothermic equilibrium: [Co(H2O) 6]Cl2 (aq) ⇌ [CoCl2(H2O) 4] (aq) + 2 H2O (l) (colorless) (blue) If the reactant solution is used to write on a piece of paper and the paper is allowed to partially dry,what can be done to bring out the colored handwriting?
A) add water
B) decrease the volume
C) put the paper in a freezer
D) put the paper in an oven
Correct Answer

verified
Correct Answer
verified
Multiple Choice
"If a stress is applied to a reaction mixture at equilibrium,the reaction occurs in the direction that will relieve the stress." This statement is called
A) the Third Law of Thermodynamics.
B) the Law of Combining Volumes.
C) the Law of Mass Action.
D) Le Châtelier's principle.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the hypothetical reaction: 2 A(s) + x B(g) ⇌ 3 C(g) ,Kp = 0.0105 and Kc = 0.45 at 250°C.What is the value of the coefficient x?
A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Shown below is a concentration vs.time plot for the reaction A ⇌ B.For this reaction the value of the equilibrium constant is 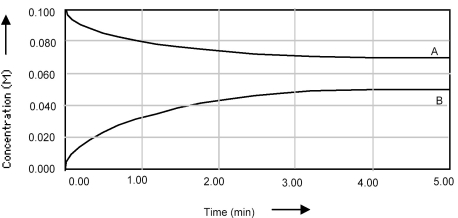
A) Kc < 1.
B) Kc = 0.
C) Kc = 1.
D) Kc > 1.
Correct Answer

verified
Correct Answer
verified
Short Answer
The reaction CaCO3(s)⇌ CaO(s)+ O2(g)is endothermic 298 K.The effect of adding a catalyst to the system at equilibrium will ________ (decrease,increase,have no effect on)the total quantity of CaCO3 once equilibrium is reestablished.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Find the equilibrium constant for the reaction: A(g) + B(g) ⇌ 2C(g) at 25°C when k equals 1.4 × 10-12 M-1s-1 for the reaction A(g) + B(g) → 2C(g) at 25°C and k equals 2.7 × 10-13 M-1s-1 for the reaction: 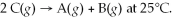
A) 3.8 × 10-25
B) 1.7 × 10-12
C) 1.1 × 10-12
D) 5.2
Correct Answer

verified
Correct Answer
verified
Showing 141 - 160 of 171
Related Exams