A) They both consist of atoms from different elements.
B) The way in which their atoms are bonded together.
C) One is a solid and the other is a liquid.
D) The components of a mixture are not chemically bonded together.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The oldest known elements in the periodic table are the ones ________.
A) with the lowest atomic numbers
B) with the highest atomic numbers
C) that match their Latin names
D) with atomic symbols that match their modern names
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The repeating trends that take place when examining the elements are called ________.
A) periodicity
B) the family cycle
C) the metal shift
D) a group conscience
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are metalloids?
A) elements that have some properties like metals and some like nonmetals
B) elements that are smaller than metals
C) elements found in asteroids
D) elements that are larger than nonmetals
E) elements that have properties different than either the metals or the nonmetals
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What do chicken noodle soup and garden soil have in common?
A) They are both examples of heterogeneous mixtures.
B) They both contain elements.
C) They are both examples of compounds.
D) nothing
Correct Answer

verified
Correct Answer
verified
Multiple Choice
About how many elements do you have access to as a consumer of market goods?
A) none
B) one
C) ten
D) one hundred
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following image represents which kind of matter? 
A) a mixture
B) a compound
C) an element
D) none of the above
E) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How would you classify the following material? coffee (black)
A) a suspension
B) a heterogeneous mixture
C) a solution
D) an element
E) a compound
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If you eat metallic sodium or inhale chlorine gas, you stand a strong chance of dying. Let these two elements react with each other, however, and you can safely sprinkle the compound on your popcorn for better taste. What is going on?
A) After these two elements react they lose the potential energy to cause harm.
B) All elements are inherently dangerous
C) Sodium and chlorine from the elemental form is more concentrated than the sodium and chlorine we get from sodium chloride.
D) Sodium chloride has nothing in common with the properties of the elements sodium and chlorine.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Is the following transformation representative of a physical change or a chemical change? 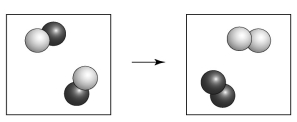
A) chemical change because of the formation of elements
B) physical change because a new material has been formed
C) chemical change because the atoms are connected differently
D) physical change because of a change in phase
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a metalloid?
A) antimony (atomic no. = 51)
B) zinc (atomic no. = 30)
C) iodine (atomic no. = 53)
D) uranium (atomic no. = 92)
E) sulfur (atomic no. = 16)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Half-frozen fruit punch is always sweeter than the same fruit punch completely melted because ________.
A) the sugar sinks to the bottom
B) crystallization is a purifying process
C) the half-frozen fruit punch is warmer
D) sugar molecules are less soluble in a half-frozen solution
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement best describes a compound?
A) a material that is made up of a combination of atoms bonded together
B) a mixture of more than one element
C) a mixture of atoms
D) a material that is made up of a single type of atom
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Strontium, Sr (number 38) , is especially dangerous to humans because it tends to accumulate in calcium-dependent bone marrow tissues (calcium, Ca, number 20) . This fact relates to the organization of the periodic table in that strontium and calcium are both ________.
A) metals
B) in group 2 of the periodic table
C) made of relatively large atoms
D) soluble in water
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The probe tip of a scanning probe microscope ________.
A) floats above the atoms of a sample
B) etches into the atoms of a sample
C) chemically changes the atoms of a sample
D) injects a magnetic field into the atoms of a sample
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Elements that are in the same ________ have a tendency to have very similar chemical properties due to periodic trends.
A) group
B) period
C) textbook
D) compound
E) row
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If you filter sea water to remove all of the particles you would be left with a clear ________.
A) homogeneous mixture called a solution
B) homogeneous mixture called a suspension
C) heterogeneous mixture called a solution
D) heterogeneous mixture called a suspension
E) pure liquid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Classify the following changes as physical or chemical. Wood burns to ashes; water begins to boil; grass grows; a rock is crushed to powder.
A) chemical; physical; chemical; chemical
B) chemical; physical; physical; physical
C) physical; physical; chemical; physical
D) chemical; physical; chemical; physical
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many atoms are in one molecule of Na2SO4?
A) 7
B) 2
C) 4
D) 3
E) 24
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the difference between a compound and a mixture?
A) A mixture can be physically separated into its components; a compound cannot be physically separated into its components.
B) A compound can be physically separated into its components; a mixture cannot be physically separated into its components.
C) A compound is just a mixture of elements.
D) The components of a mixture do not have the same properties individually as they do when mixed.
E) The components of a compound have the same properties individually as they do when mixed.
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 116
Related Exams