A) 97.2 nm
B) 82.6 nm
C) 365 nm
D) 0.612 nm
E) 6.8 * 10-18 nm
Correct Answer

verified
Correct Answer
verified
Short Answer
Write the ground state electron configuration for Ni.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Electrons in an orbital with l = 3 are in a/an
A) d orbital.
B) f orbital.
C) g orbital.
D) p orbital.
E) s orbital.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A ground-state atom of vanadium has ___ unpaired electrons and is _____.
A) 0, diamagnetic
B) 2, diamagnetic
C) 3, paramagnetic
D) 5, paramagnetic
E) 4, diamagnetic
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the ground-state electron configuration of a calcium atom?
A) [Ne]3s2
B) [Ne]3s23p6
C) [Ar]4s13d1
D) [Ar]4s2
E) [Ar]3d2
Correct Answer

verified
Correct Answer
verified
Short Answer
Which one of the following sets of quantum numbers is not possible? 
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Complete this sentence: Atoms emit visible and ultraviolet light
A) as electrons jump from lower energy levels to higher levels.
B) as the atoms condense from a gas to a liquid.
C) as electrons jump from higher energy levels to lower levels.
D) as they are heated and the solid melts to form a liquid.
E) as the electrons move about the atom within an orbit.
Correct Answer

verified
Correct Answer
verified
Short Answer
An AM radio station broadcasts at a frequency of 1270 kHz.Calculate the wavelength of the broadcast signal in meters.(c = 2.9979 * 108 m/s)
Correct Answer

verified
Correct Answer
verified
Short Answer
An FM radio station broadcasts at a frequency of 101.7 MHz.Calculate the wavelength of the broadcast signal in meters.(c = 2.9979 * 108 m/s)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Electrons can be used to probe the arrangement of atoms on a solid surface if the wavelength of the electrons is comparable with the spacing between the atoms. Which of the following electron velocities would be appropriate for use in this application if the atoms are separated by 0.320 nm?
A) 2.27 * 106 m/s
B) 1.24 * 103 m/s
C) 3.00 * 108 m/s
D) 4.41 * 106 m/s
E) 8.06 * 103 m/s
Correct Answer

verified
Correct Answer
verified
Short Answer
The orbital diagram for a ground state carbon atom is 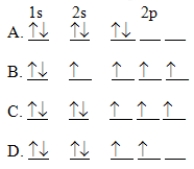
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the binding energy (in J/mol or kJ/mol) of an electron in a metal whose threshold frequency for photoelectrons is 2.50 * 1014 /s?
A) 99.7 kJ/mol
B) 1.66 * 10-19 J/mol
C) 2.75 * 10-43 J/mol
D) 7.22 * 1017 kJ/mol
E) 1.20 * 10-6 J/mol
Correct Answer

verified
Correct Answer
verified
True/False
According to de Broglie's equation, the wavelength associated with the motion of a particle increases as the particle mass decreases.
Correct Answer

verified
Correct Answer
verified
Short Answer
Write the ground state electron configuration for an iodine atom.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element has the following ground-state electron configuration? [Kr]5s24d105p2
A) Sn
B) Sb
C) Pb
D) Ge
E) Te
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the total number of electrons possible in the 2p orbitals?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the frequency of the light emitted by a hydrogen atom during a transition of its electron from the n = 6 to the n = 3 principal energy level. Recall that for hydrogen En = -2.18 * 10-18 J(1/n2) .
A) 1.64 * 1015 /s
B) 9.13 * 1013 /s
C) 3.65 * 1014 /s
D) 1.82 * 10-19 /s
E) 2.74 * 1014/s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The longest wavelength of light that causes electrons to be ejected from the surface of a copper plate is 243 nm. What is the maximum velocity of the electrons ejected when light of wavelength 200.nm shines on a copper plate?
A) 1.48 * 106 m/s
B) 6.22 * 105 m/s
C) 4.67 * 104 m/s
D) 1.97 * 104 m/s
E) 1.34 * 106 m/s
Correct Answer

verified
Correct Answer
verified
True/False
The frequency of the emitted light from a cesium atom is an intensive property.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the energy in joules of a mole of photons associated with red light of wavelength 7.00 * 102 nm?
A) 256 kJ
B) 1.71 * 105 J
C) 4.72 * 10-43 J
D) 12.4 kJ
E) 2.12 * 1042 J
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 115
Related Exams