A) hexagonal close-packed
B) cubic close-packed
C) body centered
D) simple
E) all of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
Crystals of elemental sulfur are easily crushed, and melt at 113°C.Liquid sulfur does not conduct electricity.What kind of crystal is this?
Correct Answer

verified
Correct Answer
verified
Short Answer
Magnesium oxide, MgO, melts at 2,800°C and is very hard.The liquid conducts electricity very well.What kind of crystal is this?
Correct Answer

verified
Correct Answer
verified
Short Answer
Which would have the higher boiling point CH3Cl or CH4?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which property of water allows a razor blade to float on it without sinking?
A) viscosity
B) surface tension
C) density
D) specific heat
E) triple point
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the graph of vapor pressure to determine the normal boiling point of CHCl3. 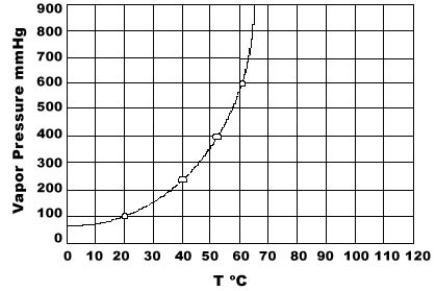
A) 19°C
B) 52°C
C) 60°C
D) 64°C
E) 70°C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of atoms in a face-centered cubic unit cell is
A) 1
B) 2
C) 3
D) 4
E) 8
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molar enthalpy of vaporization of hexane (C6H14) is 28.9 kJ/mol, and its normal boiling point is 68.73°C.What is the vapor pressure of hexane at 25°C?
A) 4.44 torr
B) 117 torr
C) 171 torr
D) 759 torr
E) 3370 torr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The zincblende structure of ZnS has the relatively large sulfide ions arranged at the lattice points of a face-centered cubic structure.The edge length of this cubic unit cell is 540.9 pm.Determine the density of zincblende.
A) 1.023 g/cm3
B) 2.032 g/cm3
C) 2.046 g/cm3
D) 3.081 g/cm3
E) 4.091 g/cm3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The intermolecular forces present in HSCH2CH2SH include which of the following? I.dipole-dipole II.ion-dipole III.dispersion IV.hydrogen bonding
A) I, II, III, and IV
B) I and III
C) I, III, and IV
D) I and II
E) II and IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following phase changes is endothermic?
A) Sublimation
B) Condensation
C) Freezing
D) Deposition
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The intermolecular forces present in CH3NH2 include which of the following? I.dipole-dipole II.ion-dipole III.dispersion IV.hydrogen bonding
A) I, II, III, and IV
B) I and III
C) I, III, and IV
D) I and II
E) II and IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The triple point of iodine is at 0.12 atm and 115°C.Thus, liquid I2
A) is more dense than I2 (s) .
B) cannot exist above 115°C.
C) is liquid at room temperature.
D) cannot have a vapor pressure less than 91 torr.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A liquid boils when its
A) vapor pressure is exactly 1 atmosphere.
B) vapor pressure is equal to, or greater than, the external pressure pushing on it.
C) temperature is equal to 273 K (standard temperature) .
D) temperature is greater than room temperature.
Correct Answer

verified
Correct Answer
verified
Short Answer
Calculate the amount of enthalpy required to heat 25.0 g of solid benzene (C6H6)at -10°C to liquid benzene at 20.0°C.Thermodynamic data for benzene: specific heat of solid benzene = 1.52 J/g·°C; specific heat of liquid benzene = 1.73 J/g·°C; melting point = 5.5°C; Hfus = 9.9 kJ/mol.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on the phase diagram shown below, how will the melting point of the substance change if the pressure is increased above 1 atm? 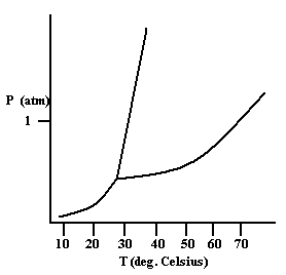
A) The melting point will decrease.
B) The melting point will remain the same.
C) The melting point will increase.
D) The substance will not melt at pressures of 1 atm and above; instead, the solid sublimes to form the gas phase.
Correct Answer

verified
Correct Answer
verified
Short Answer
Osmium tetroxide, OsO4, is a soft crystal that melts at 40°C.The liquid does not conduct electricity.What kind of crystal is this?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Find the temperature at which water boils on a day in the mountains when the barometric pressure is 593 mmHg.(Given: the heat of vaporization of water is 40.79 kJ/mol)
A) 41.5°C
B) 68.1°C
C) 93.1°C
D) 97.0°C
E) 117°C
Correct Answer

verified
Correct Answer
verified
Essay
Suppose the atoms in a two-dimensional crystal have the following arrangement: 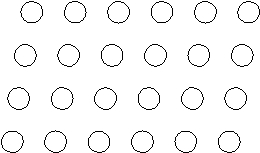 On the drawing above, sketch the unit cell of this crystal.
On the drawing above, sketch the unit cell of this crystal.
Correct Answer

verified
Correct Answer
verified
Short Answer
Iron crystallizes in a body-centered cubic unit.The edge of this cell is 287 pm.Calculate the density of iron.
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 149
Related Exams