A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer

verified
Correct Answer
verified
Short Answer
According to the VSEPR theory, will the molecule PF5 will be polar or nonpolar?
Correct Answer

verified
Correct Answer
verified
Essay
How does the geometrical structure of PF5 differ from that of IF5?
Correct Answer

verified
PF5 is trigonal bipyr...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever.What is the hybridization state of carbon indicated by the arrow in the structure of ibuprofen shown below? 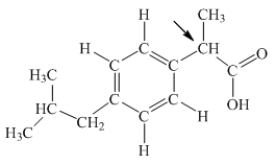
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Indicate the type of hybrid orbitals used by the central atom in PCl3.
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer

verified
Correct Answer
verified
True/False
The geometry of the hybrid orbitals of the central atom of a molecule is always the same as molecular geometry.
Correct Answer

verified
Correct Answer
verified
Short Answer
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. 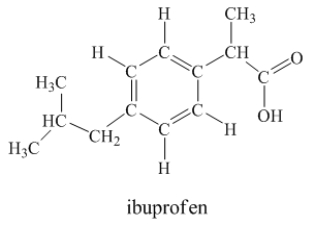 How many sigma bonds and pi bonds are contained in an ibuprofen molecule?
How many sigma bonds and pi bonds are contained in an ibuprofen molecule?
Correct Answer

verified
33 sigma b...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which one of the following molecules has a zero dipole moment?
A) CO
B) CH2Cl2
C) SO3
D) SO2
E) NH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule with 2 single bonds and 3 lone pair of electrons is predicted to have which type of moleculary geometry?
A) Octahedral
B) T-shaped
C) Seesaw
D) Trigonal bipyramidal
E) Linear
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The electrons in the delocalized molecular orbitals of benzene (C6H6)
A) are confined between two adjacent bonding atoms.
B) are free to move around the six-membered ring.
C) form the electron pairs in the C-H bonds of the compound.
D) are unevenly distributed through the molecule.
E) are responsible for the fact that the bonds between three pairs of carbon atoms in the ring are longer and stronger than the bonds between the other three pairs of carbon atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sp hybridized terminal nitrogen atom (bonded to one other atom only) with 1 lone pair of electrons has what type of bonding?
A) 1 and 2 bonds
B) 2 and 1 bonds
C) 2 and 2 bonds
D) 0 and 2 bonds
E) 0 and 3 bonds
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The geometry of the SF4 molecule is
A) tetrahedral.
B) trigonal pyramidal.
C) trigonal planar.
D) square planar.
E) distorted tetrahedron (seesaw) .
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to Molecular Orbital Theory, two separate px orbitals interact about the y-axis to form what molecular orbitals?
A) ( and *)
B) ( and *)
C) ( , * and )
D) ( , *, and )
E) ( , *, , and *)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species has the largest dipole moment (i.e., is the most polar) ?
A) CH4
B) CH3Br
C) CH3Cl
D) CH3F
E) CH3I
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules have the same geometries?
A) SF4 and CH4
B) CO2 and H2O
C) CO2 and BeH2
D) N2O and NO2
Correct Answer

verified
Correct Answer
verified
True/False
The BrF5 molecule has polar bonds and has a net dipole moment.
Correct Answer

verified
Correct Answer
verified
Essay
Explain why CO2 is nonpolar, but OCS is polar.
Correct Answer

verified
In CO2 the two bond moments point in oppo...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
According to the VSEPR theory, which one of the following species is linear?
A) H2S
B) HCN
C) BF3
D) H2CO
E) SO2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use VSEPR theory to predict the geometry of the PCl3 molecule.
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the VSEPR theory, the geometry of the SO3 molecule is
A) pyramidal.
B) tetrahedral.
C) trigonal planar.
D) distorted tetrahedron (seesaw) .
E) square planar.
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 147
Related Exams