Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true of a buffer prepared with equal concentrations of an acid and its conjugate base?
A) pH = 7
B) pH = pKa
C) The pH depends on the concentration of the buffer.
D) none of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
The buffer solution in the flask was prepared using one of the substances shown below.
citric acid (pKa 3.08)
NaH2PO4 (pKa 7.21)
NH4Cl (pKa 9.25)
Using the pH meter and solution shown in the image below,  complete the following statements using one of the terms below.
increase
decrease
remain constant
citric acid
NaH2PO4
NH4Cl
-The buffer solution in the beaker could be prepared using__________.
complete the following statements using one of the terms below.
increase
decrease
remain constant
citric acid
NaH2PO4
NH4Cl
-The buffer solution in the beaker could be prepared using__________.
Correct Answer

verified
Correct Answer
verified
True/False
Consider the following reaction.
CH3OH(l) + CH3OH(l) 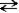 CH3O-(l) + CH3OH2+(l)
This reaction could be classified as both an autoionization reaction and a proton transfer reaction.
CH3O-(l) + CH3OH2+(l)
This reaction could be classified as both an autoionization reaction and a proton transfer reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following will determine the pH of a buffer made using acetic acid (HC2H3O2) and sodium acetate (NaC2H3O2) ?
A) the amount of water present
B) the concentration of C2H3O2-
C) the concentration of HC2H3O2
D) the ratio of the concentrations of C2H3O2- and HC2H3O2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following substances can react with both HCl and NaOH?.
A) Ca(HCO3) 2
B) KCl
C) CH3CH2NH2
D) H2C2O4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is characteristic of a buffer?
A) The pH will go down significantly when H3O+ is added to the buffer.
B) The pH will go down very slightly when H3O+ is added to a buffer.
C) The pH will go up significantly when H3O+ is added to the buffer.
D) The pH will go up very slightly when H3O+ is added to the buffer.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a solution of HF (a weak acid) is added to a solution containing HPO42- (here, a base) , the equation for reaction that occurs is:
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Short Answer
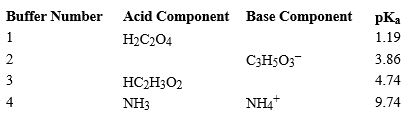 Using the above table,fill in the blank with the appropriate integer (1 ,2,3,...)indicating the buffer and/or the letter indicating the formula below.
-Buffer ______________________ would be the most effective buffer in basic solutions.
A)H3C2O4+
B)HC2O4-
C)C3H4O32-
D)HC3H5O3
E)C2H3O2-
F)H2C2H3O2
G)C3H5O3-
Using the above table,fill in the blank with the appropriate integer (1 ,2,3,...)indicating the buffer and/or the letter indicating the formula below.
-Buffer ______________________ would be the most effective buffer in basic solutions.
A)H3C2O4+
B)HC2O4-
C)C3H4O32-
D)HC3H5O3
E)C2H3O2-
F)H2C2H3O2
G)C3H5O3-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The chemical equation for the reaction of the base, ethoxide, CH3CH2O-, with water would show the products:
A) CH3CH2OH and OH-
B) CH3CH3 and H3O+
C) CH3CH2OH and H3O+
D) CH3CH3 and OH-
Correct Answer

verified
Correct Answer
verified
True/False
A solution composed of HCl and NaCl would produce an effective buffer.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an aqueous solution. the [OH-] is 2.0 × 10-3 M. What is the [H3O+] of this solution?
A) 5.0 × 10-12 M
B) 2.0 × 1011 M
C) 2.0 × 10-3 M
D) 5.0 × 1012 M
Correct Answer

verified
Correct Answer
verified
Short Answer
NaOH and H2SO4 are mixed and allowed to react. How many grams of NaOH are required to neutralize 17.25 g of H2SO4?
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the following three buffer reactions. Buffer 1: protein-H+ and protein Buffer 2: H2CO3 and HCO3- Buffer 3: H2PO4- and HPO42- Fill in the blank with the appropriate integer (1,2,or 3)to indicate the buffer system described. -Buffer__________________is found in both plasma and red blood cells.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following solutions can function as a buffer?
A) a solution containing HC2H3O2 and NaC2H3O2
B) a solution containing H2CO3 and NaHCO3
C) a solution containing NH3 and NH4Cl
D) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is most likely to be a salt?
A) Ca(NO3) 2
B) CH3Cl
C) CH3CH2NH2
D) H2C2O4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is excreted by the kidneys to regulate the effect of excess protein in the diet?
A) NH4+
B) H3O+
C) HCO3-
D) H2PO4-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction.
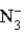 + H2O
+ H2O 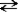 HN3 + OH−
This reaction indicates that:
HN3 + OH−
This reaction indicates that:
A) ![]() is a weak base.
is a weak base.
B) ![]() is a strong base.
is a strong base.
C) ![]() is a weak acid.
is a weak acid.
D) ![]() is a strong acid.
is a strong acid.
Correct Answer

verified
Correct Answer
verified
True/False
Adipic acid has the formula given below. 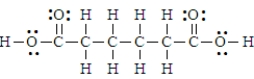 Thus substance would be classified as amphiprotic.
Thus substance would be classified as amphiprotic.
Correct Answer

verified
Correct Answer
verified
True/False
In converting from the pH of a solution to the corresponding H3O+ concentration, the following equation should be used. [H3O+] = 10-pH
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 82
Related Exams