A) 37.2 % (v/v)
B) 269% (v/v)
C) 0.372% (v/v)
D) 27.1% (v/v)
Correct Answer

verified
Correct Answer
verified
True/False
In cases of cerebral endema, a hypertonic solution is administered. The goal of giving the patient the hypertonic solution would be to pull fluid from the cells through the process of osmosis.
Correct Answer

verified
Correct Answer
verified
True/False
Solution contains 55 mg of magnesium in 2.5 L of solution. The concentration of this solution is 2.2 mg/dL.
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider two solutions,A and B,separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below. 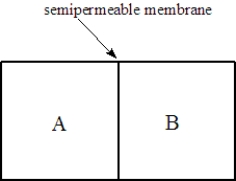 Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
-Solution A is 0.50 M in sucrose and Solution is 1.5 M in sucrose. After one hour, the compartment on the ____________________ will have the higher osmotic pressure.
Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
-Solution A is 0.50 M in sucrose and Solution is 1.5 M in sucrose. After one hour, the compartment on the ____________________ will have the higher osmotic pressure.
Correct Answer

verified
Correct Answer
verified
Short Answer
For the following questions,fill in the blank with one of the following terms as appropriate. increase decrease remains constant cannot predict -As the size of the hydrophobic portion of a molecule increases, the solubility in water will ___________.
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider two solutions,A and B,separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below.  Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
-Solution A is pure water, and solution B is 0.05 M glucose. Water molecules will move to the ______________________compartment and glucose molecules will move to the __________________compartment.
Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
-Solution A is pure water, and solution B is 0.05 M glucose. Water molecules will move to the ______________________compartment and glucose molecules will move to the __________________compartment.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of solid KCl are needed to prepare 250.0 mL of 0.235 M solution?
A) 9.32 g
B) 31.3 g
C) 15.6 g
D) 4.38 g
Correct Answer

verified
Correct Answer
verified
Short Answer
For the following questions,fill in the blank with one of the following terms as appropriate. increase decrease remains constant cannot predict -For many ionic solids such as NaHCO3 water solubility will ___________ at higher temperatures..
Correct Answer

verified
Correct Answer
verified
True/False
The osmotic pressure of a 0.10 M NaCl solution will be the same as that of a 0.10 M urea solution.
Correct Answer

verified
Correct Answer
verified
Essay
Write the conversion factor that corresponds to 7.75%(w/v) NaCl.
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the two containers separated by a semipermeable membrane that allows both glucose and sucrose to pass through.  Answer the following questions as appropriate with: right,left,or no movement.
-Upon standing the glucose molecules will move in which direction?
Answer the following questions as appropriate with: right,left,or no movement.
-Upon standing the glucose molecules will move in which direction?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution has a concentration of 16 ppm. Which of the following is another way to describe the concentration of this solution?
A) 0.016 ppb
B) 0.16 ppb
C) ![]() ppb
ppb
D) ![]() ppb
ppb
Correct Answer

verified
Correct Answer
verified
True/False
Consider the following graph. 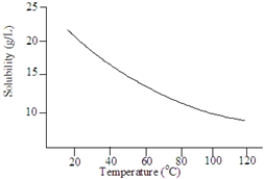 The solute is probably a gas.
The solute is probably a gas.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution is made by dissolving 5.84 grams of NaCl in enough distilled water to give a final volume of 1.00 L. What is the molarity of the solution?
A) 0.100 M
B) 1.00 M
C) 0.0250 M
D) 0.400 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar mass of ibuprofen, C13H18O2?
A) 29.0 g/mol
B) 206.3 g/mol
C) 289.4 g/mol
D) 377.7 g/mol
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the following structure. 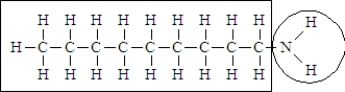 Complete the sentence using the appropriate terms given below.
hydrophobic
hydrophilic
soluble
insoluble
-This molecule is probably____________________in water.
Complete the sentence using the appropriate terms given below.
hydrophobic
hydrophilic
soluble
insoluble
-This molecule is probably____________________in water.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following list gives the concentration of the major components of blood plasma.
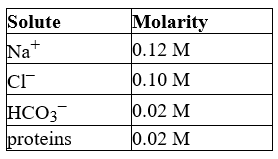 What is the total concentration of all the minor components in the plasma?
What is the total concentration of all the minor components in the plasma?
A) 0.02 M
B) 0.28 M
C) 0.12 M
D) Cannot be determined with the given information.
Correct Answer

verified
Correct Answer
verified
True/False
The solubility of a compound in water was measured and found to be 0.7 g/L. This compound would be classified as insoluble.
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider two solutions,A and B,separated by a seminpermeable membrane that allows water and small molecules to pass through as shown below.  Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
-Solution A is 0.10 M in fructose and 0.05 M in glucose. Solution B is 0.20 M in sucrose. The direction of osmosis will be to the _______________________.
Fill the blank(s)with the appropriate terms from the list below.
right
left
osmosis
dialysis
-Solution A is 0.10 M in fructose and 0.05 M in glucose. Solution B is 0.20 M in sucrose. The direction of osmosis will be to the _______________________.
Correct Answer

verified
Correct Answer
verified
Short Answer
The vitamins A (retinol)and C (ascorbic acid)are shown below.All atoms other than C and H are explicitly shown. 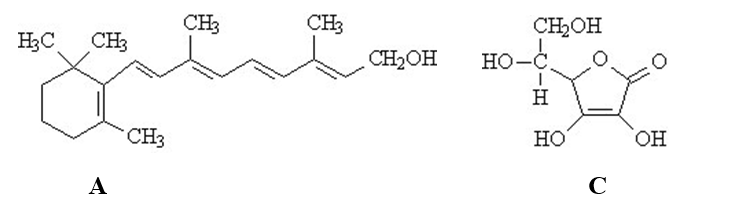 Complete the following questions be entered in the appropriate letter (A or C)in the blank provided.
-The beaker below contains oil (a compound of mostly carbon and hydrogen) in the upper layer and water in the lower layer. Oil floats on top of the water because it is less dense.
Complete the following questions be entered in the appropriate letter (A or C)in the blank provided.
-The beaker below contains oil (a compound of mostly carbon and hydrogen) in the upper layer and water in the lower layer. Oil floats on top of the water because it is less dense.  The vitamin that would be the most soluble in the upper layer is ______.
The vitamin that would be the most soluble in the upper layer is ______.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 76
Related Exams