Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the major product for the reaction: 1-butene + HI →
A) CH2ICH2CH2CH3
B) CH2ICH=CHCH3
C) CH2=CICH2CH3
D) CH3CHICH2CH3
E) CH3CI2CH2CH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following are nucleophiles? I.CH3COCH3 II.CH3Br III.CH3CH2C≡C-
A) I
B) II
C) III
D) I + II
E) I + III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict major products for chlorination of the following three aromatic compounds: 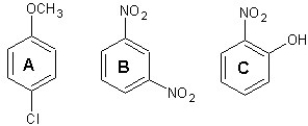
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Classify the following reaction steps as initiation,propagation or termination. A: Br-Br → 2 Br∙ B: H3CH2C∙ + Br∙ → CH3CH2Br C: 2 H3CH2C∙ → CH3CH2CH2CH3
A) Step A is initiation and steps B and C are propagation
B) Steps A and B are initiation and step C is termination
C) Step A is initiation,step B is termination and step C is propagation
D) Step A is initiation,step B is propagation and step C is termination
E) Step A is initiation and steps B and C are termination
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following steps can be found in any of the polymerization mechanisms you learned? I.initiation II.condensation III.propagation IV.dehydrohalogenation V.rearrangement
A) I,III,V.
B) II,III and IV
C) III,IV,and V
D) I,II,and III
E) II,III,and V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When (CH3) 3CBr reacts with CH3ONa in methanol,two products are formed: a major one is an alkene and minor one is an ether.Identify the two products and by what mechanism(s) they are formed?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Complete the following reaction: NH3 + CH3Br →
A) CH4 + NH2Br
B) CH2=NH + H2 + HBr
C) CH3NH2 + NH4+Br-
D) CH3NH3+Br-
E) There is no reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which reagents would you choose to carry out the following transformations?
I. II.
II. 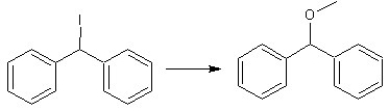 III.
III. 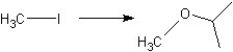
A) Reaction I use KOtBu
Reaction II use MeOH
Reaction III use NaOCH(CH3) 2
B) Reaction I use NaOMe
Reaction II use MeOH
Reaction III use NaOC(CH3) 3
C) Reaction I use NaOMe
Reaction II use MeOH
Reaction III use NaOMe
D) Reaction I use KOtBu
Reaction II use HC(=O) H
Reaction III use NaOCH(CH3) 2
E) Reaction I use KOtBu
Reaction II use Na2Cr2O7 or K2Cr2O7
Reaction III use NaOCH(CH3) 2
Correct Answer

verified
Correct Answer
verified
True/False
Synthesis of alkanes from alkyl halides is a typical example of E2 reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the product for the following reaction: 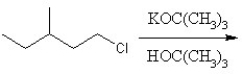
A) 3-methyl-1-pentene
B) 3-methyl-2-pentene
C) 3-methyl-1-pentane
D) 3-methyl-1-tert-butylpentante
E) 3-methylpentane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate of E2 reaction between an alkyl halide RX and a base B is given by the following expression (k is a rate constant) :
A) k[RX]
B) k[B]
C) k[RX][B]
D) k[RX]2
E) k[RX]2[B]
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following groups would be ortho,para directors in electrophilic aromatic substitution? I.-CN II.-SO3H III.-NH2 IV.-OH V.-Br
A) III ,IV ,and V
B) II ,III ,and V
C) I ,III ,and IV
D) I ,II and IV
E) II ,IV and V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Finish the sentence: "Elimination reactions...
A) ...can follow two different mechanisms."
B) ...do not compete with other reaction types."
C) ...usually give a saturated product."
D) ...always give only one product."
E) ...have only one mechanistic path."
Correct Answer

verified
Correct Answer
verified
Showing 81 - 94 of 94
Related Exams