A) zero
B) first
C) second
D) third
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction: C2H4Br2 + 3 KI → C2H4 + 2 KBr + KI3,when the rate of disappearance of C2H4Br2 is 2.0 × 10-5 M/s,what is the rate of disappearance of KI?
A) 0.67 × 10-5 M/s
B) 2.0 × 10-5 M/s
C) 4.0 × 10-5 M/s
D) 6.0 × 10-5 M/s
E) 1.0 × 10-5 M/s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction A + B → C + D is second order in A and zero order in B.The value of k is 0.012 M-1 min-1.What is the rate of this reaction when [A] = 0.125 M and [B] = 0.435 M?
A) 5 × 10-4 M min-1
B) 3.4 × 10-3 M min-1
C) 1.3 M min-1
D) 1.9 × 10-4 M min-1
E) 1.5 × 10-3 M min-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct units of the specific rate constant for a zero order reaction are ________.
A) L/mol s
B) s-1
C) s
D) L2/mol2 s
E) rate is a constant,so it has no units
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The first-order reaction,2 N2O(g) → 2 N2(g) + O2(g) ,has a rate constant equal to 0.76 s-1 at 1000 K.How long will it take for the concentration of N2O to decrease to 12% of its initial concentration?
A) 0.62 s
B) 2.8 s
C) 6.3 s
D) 8.4 s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is rate = k[HgCl2] 2[C2O42-] not the rate law for the following mechanism? 2 HgCl2 + C2O42- → 2 Cl- + 2 CO2 + Hg2Cl2 (overall reaction) HgCl2 + C2O42- ⇌ HgCl2C2O42- (fast) HgCl2C2O42- + C2O42- → Hg + 2 C2O4Cl2- (slow) Hg + HgCl2 → Hg2Cl2 (fast) 2 C2O4Cl2- → C2O42- + 2 Cl- + 2 CO2 (fast)
A) The rate law is not based on the slow step of the proposed mechanism.
B) The steps do not add to the overall reaction.
C) The rate law does not agree with the overall reaction.
D) The exponents of HgCl2 and C2O42- are not equal.
E) The first step is not the slow step.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A particular first-order reaction has a rate constant of 1.35 × 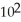
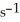 At 25.0 °C.What is the magnitude of k at
At 25.0 °C.What is the magnitude of k at 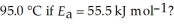
A) 9.56 × 103 s-1
B) 2.85 × 104 s-1
C) 576 s-1
D) 4.33 × 1087 s-1
E) 1.36 × ![]() s-1
s-1
Correct Answer

verified
Correct Answer
verified
True/False
In the Arrhenius equation,ln k = -Ea/RT + ln A,the symbol A denotes the initial concentration of A.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement is INCORRECT?
A) Activation energy is always the same for forward and reverse reaction.
B) If the forward reaction is endothermic,the reverse will be exothermic.
C) In an endothermic reaction,activation energy is usually greater than the enthalpy.
D) An activated complex has higher energy than any molecule contributing to it.
E) The activated complex will be the highest on the energy profile.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The first-order reaction,SO2Cl2 → SO2 + Cl2,has a rate constant equal to 2.20 × 10-5 s-1 at 593 K.What percentage of the initial amount of SO2Cl2 will remain after 6.00 hours?
A) 1.00%
B) 37.8%
C) 40.2%
D) 62.2%
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate data from a chemical reaction shows that doubling the concentration of A with the concentration of B remaining constant causes the rate to increase by a factor of four.What is the reaction order for [A]?
A) 0
B) 0.5
C) 1
D) 2
E) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The combustion of ethylene proceeds by the reaction
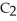
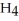 (g) +
(g) + 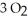 (g) →
(g) → 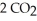 (g) +
(g) + 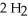 O(g)
When the rate of disappearance of
O(g)
When the rate of disappearance of 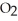 Is 0.28 mol L-1
Is 0.28 mol L-1 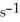 ,the rate of appearance of
,the rate of appearance of 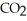 Is ________
Is ________ 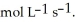
A) 0.19
B) 0.093
C) 0.84
D) 0.42
E) 0.56
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following initial rate data,calculate the specific rate constant. 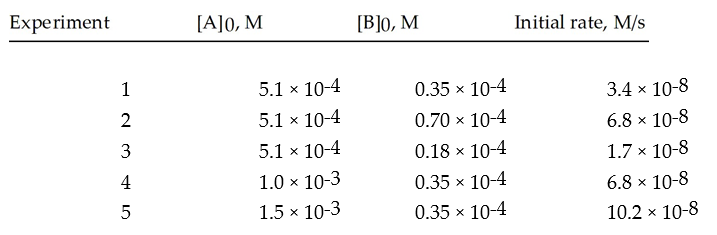
A) 1.9 M2/s2
B) 1.9 M-1 s-1
C) 3.6 M s-1
D) 1.1 × 108 M2/s2
E) 3.6 M2 s-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate rate constant k for a first order reaction with a half-life of 75.0 min.
A) 52.0 min-1
B) 1.54 × 10-4 min-1
C) 1.33 × 10-2 min-1
D) 9.24 × 10-3 min-1
E) 2.67 × 10-2 min-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction: C2H4Br2 + 3 KI → C2H4 + 2 KBr + KI3
Initial rate data at 60 °C are:
![For the reaction: C<sub>2</sub>H<sub>4</sub>Br<sub>2</sub> + 3 KI → C<sub>2</sub>H<sub>4</sub> + 2 KBr + KI<sub>3</sub> Initial rate data at 60 °C are: The rate law is ________. A) rate = k[KI] B) rate = k[C<sub>2</sub>H<sub>4</sub>Br<sub>2</sub>] C) rate = k[KI]<sup>2 </sup> D) rate = k[KI][C<sub>2</sub>H<sub>4</sub>Br<sub>2</sub>] E) rate = k[KI][C<sub>2</sub>H<sub>4</sub>Br<sub>2</sub>]<sup>2 </sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB5343/11ec76bd_e061_92de_9e6f_23382285cb36_TB5343_00.jpg) The rate law is ________.
The rate law is ________.
A) rate = k[KI]
B) rate = k[C2H4Br2]
C) rate = k[KI]2
D) rate = k[KI][C2H4Br2]
E) rate = k[KI][C2H4Br2]2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the rate law for the following mechanism? N2O + NO → N2ONO (Slow) N2ONO → N2 + NO2 (Fast)
A) Rate = k[N2O]
B) Rate = k[NO]
C) Rate = k[N2O][NO]
D) Rate = k[N2][NO2]
E) Rate = k[N2ONO]
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the half-life of a reaction depends on the concentration of the reactant,then the reaction cannot be ________ order.
A) second
B) zero
C) first
D) third
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction 2 HgCl2 + C2O42- → products,data are:
![For the reaction 2 HgCl<sub>2</sub> + C<sub>2</sub>O<sub>4</sub><sup>2-</sup> → products,data are: The rate law is Rate = k[HgCl<sub>2</sub>]<sup>x</sup>[C<sub>2</sub>O<sub>4</sub><sup>2-</sup>]<sup>y.</sup> Thus: A) x = 2,y = 1 B) x = 2,y = 2 C) x = 1,y = 2 D) x = 1,y = 1 E) x = 0,y = 2](https://d2lvgg3v3hfg70.cloudfront.net/TB5343/11ec76bc_d8f0_03cb_9e6f_c77100f553aa_TB5343_00.jpg) The rate law is Rate = k[HgCl2]x[C2O42-]y. Thus:
The rate law is Rate = k[HgCl2]x[C2O42-]y. Thus:
A) x = 2,y = 1
B) x = 2,y = 2
C) x = 1,y = 2
D) x = 1,y = 1
E) x = 0,y = 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction: 2 N2O5(g) → 4 NO2(g) + O2(g) the rate law is:
![For the reaction: 2 N<sub>2</sub>O<sub>5</sub>(g) → 4 NO<sub>2</sub>(g) + O<sub>2</sub>(g) the rate law is: = k[N<sub>2</sub>O<sub>5</sub>] At 300 K,the half-life is 2.50 × 10<sup>4</sup> seconds and the activation energy is 103.3 kJ/mol.What is the half-life at 310 K? A) 2.49 × 10<sup>4</sup> s B) 9.51 × 10<sup>4</sup> s C) 9.51 × 10<sup>6</sup> s D) 6.57 × 10<sup>3</sup> s E) 1.87 × 10<sup>-1</sup> s](https://d2lvgg3v3hfg70.cloudfront.net/TB5343/11ea7a5e_3e7d_af43_89bd_1d21b034a742_TB5343_11.jpg) = k[N2O5]
At 300 K,the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol.What is the half-life at 310 K?
= k[N2O5]
At 300 K,the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kJ/mol.What is the half-life at 310 K?
A) 2.49 × 104 s
B) 9.51 × 104 s
C) 9.51 × 106 s
D) 6.57 × 103 s
E) 1.87 × 10-1 s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is correct?
A) A zero order reaction depends on the concentration of reactants.
B) A reaction rate cannot be calculated from the collision frequency alone.
C) The activated complex is a chemical species that can be isolated and analysed.
D) The number of collisions has no effect on the rate constant.
E) The orientation of a collision does not affect the rate constant.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 124
Related Exams