A) -276.9 kJ/mol
B) -106.1 kJ/mol
C) +171.2 kJ/mol
D) +106.1 kJ/mol
E) +138.7 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The complete combustion of propane,C3H8(g) ,is represented by the equation: C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(l) ΔrH° = -2220 kJ/mol How much heat is evolved in the complete combustion of 2.50 g C3H8(g) ?
A) 245 kJ
B) 50.4 kJ
C) 126 kJ
D) 5.56 kJ
E) 2.22 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a process at constant pressure,49,600 calories of heat are released.This quantity of heat is equivalent to:
A) 4.82 × 10-6 J
B) 1.19 × 104 J
C) 1.24 × 104 J
D) 2.08 × 105 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A certain reaction releases 10.1 kJ at constant volume and at constant pressure releases 8.4 kJ.What is △U for the reaction?
A) -10.1 kJ
B) -8.4 kJ
C) +8.4 kJ
D) -1.7 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
100.0 g of Cu (specific heat = 0.385 J/g °C) at 100.0 °C is added to 140.0 mL of H2O (specific heat = 4.184 J/g °C) at 25.0 °C.What is the final temperature of the mixture?
A) 56.3 °C
B) 29.6 °C
C) 62.5 °C
D) 91.4 °C
E) 33.7 °C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine △rH° for the decomposition of hydrogen peroxide. H2O2(l) →1/2 O2(g) + H2O(l) Given: H2(g) + O2(g) → H2O2(l) △rH° = -187.78 kJ/mol H2(g) + 1/2 O2(g) → H2O(l) △rH° = -285.83 kJ/mol
A) -98.05 kJ/mol
B) +93.89 kJ/mol
C) -191.94 kJ/mol
D) -93.89 kJ/mol
Correct Answer

verified
Correct Answer
verified
True/False
A system that absorbs heat is an exothermic system.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 5.00 g sample of liquid water at 25.0 °C is heated by the addition of 145 J of energy.The final temperature of the water is ________ °C.The specific heat capacity of liquid water is 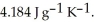
A) 146
B) 25.1
C) -18.1
D) 31.9
E) 6.94
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Cyclohexanol,C6H12O,has a heat of combustion of -890.7 kcal/mol.A sample containing 0.708 g of cyclohexanol undergoes complete combustion in a bomb calorimeter that has a heat capacity of 2.70 kcal/ °C.What is the final temperature if the initial water temperature is 27.0 °C?
A) 24.7 °C
B) 29.3 °C
C) 27.4 °C
D) 26.6 °C
E) 31.4 °C
Correct Answer

verified
Correct Answer
verified
True/False
The maximum amount of work is provided by a reversible process because equilibrium between the system and the surroundings is always maintained.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the work in joules done on the system to compress He gas from 24.0 L to 12.5 L against a pressure of 1.5 atm at a constant temperature of 37.4 °C?
A) 5.5 × 103 J
B) -17 J
C) 17 J
D) 1.7 × 103 J
E) -1.7 × 103 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given that ΔfH°[CO(g) ] = -110.5 kJ/mol and ΔfH° [COCl2(g) ] = -219.1 kJ/mol,what is the enthalpy of reaction for the formation of phosgene,COCl2 from carbon monoxide,CO,and chlorine gas,Cl2(g) ?
A) +110.5 kJ /mol
B) -110.5 kJ/mol
C) +329.6 kJ /mol
D) -108.6 kJ/mol
E) -219.1 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The specific heat capacity of methane gas is 2.20 J g-1k-1.How many joules of heat are needed to raise the temperature of 5.00 g of methane from 36.0 °C to 75.0 °C?
A) 88.6 J
B) 429 J
C) 1221 J
D) 0.0113 J
E) 22.9 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the INCORRECT statement.
A) The surroundings are the part of the universe that is studied.
B) Thermal energy is energy associated with random molecular motion.
C) Chemical energy is associated with chemical bonds and intermolecular forces.
D) Energy is the capacity to do work.
E) Work is done when a force acts through a distance.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction H2(g) + 1/2 O2(g) → H2O(g) ΔrH° = -241.8 kJ/mol, What quantity of heat is liberated by the reaction of 10.0 L of O2 measured at 22.0 °C and 742 mmHg?
A) 120 kJ
B) 2610 kJ
C) 1310 kJ
D) 195 kJ
E) 97.5 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Oxygen gas at 34.5 °C is compressed from 45.7 L to 34.5 L against a constant pressure of 750 mmHg.What is the work done in joules by the system?
A) 1.12 × 103 J
B) -1.12 × 103 J
C) 9.09 × 104 J
D) -9.09 × 104 J
E) 4.55 × 103 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Some "beetles" defend themselves by spraying hot quinone,C6H4O2(l) ,at their enemies.Calculate △rH° for this reaction. C6H4(OH) 2(l) + H2O2(l) → C6H4O2(l) + 2 H2O(l) Given: C6H4(OH) 2(l) → C6H4O2(l) + H2(g) +177.4 kJ/mol The standard enthalpies of formation of H2O2(l) and H2O(l) are -187.4 and -285.8 kJ/mol,respectively.
A) -206.8 kJ/mol
B) -384.2 kJ/mol
C) -561.6 kJ/mol
D) +79.00 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the presence of excess oxygen,methane gas burns in a constant-pressure system to yield carbon dioxide and water:
CH4(g) +2O2 (g) → CO2(g) + 2H2O (l)
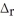 H = -890.0 kJ
Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure.
H = -890.0 kJ
Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure.
A) -105.7 kJ
B) 0.0342 kJ
C) -0.0095 kJ
D) 29.3 kJ
E) -1.06 × 105 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The specific heat capacity of liquid water is 4.184 J g-1k-1.How many joules of heat are needed to raise the temperature of 6.00 g of water from 36.0 °C to 75.0 °C?
A) 56.0 J
B) 978 J
C) 2.78 × ![]() J
J
D) 1.79 × ![]() J
J
E) 52.3 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When 1.50 mol of CH4(g) reacts with excess Cl2(g) at constant pressure according to the chemical equation shown below,1062 kJ of heat are released.Calculate the value of ΔH for this reaction,as written.
2 CH4(g) + 3 Cl2(g) → 2 CHCl3(l) + 3 H2(g)
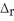 H° = ?
H° = ?
A) -1420 kJ mol-1
B) -708 kJ mol-1
C) +708 kJ mol-1
D) +1420 kJ mol-1
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 125
Related Exams