Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represents a 1.00 M aqueous solution of glucose (C6H12O6) ?
A) 90.0 g glucose per 500 mL water
B) 10.0 g glucose per 10.0 mL water
C) 0.180 g glucose per mL solution
D) 0.100 g glucose per mL solution
E) 4.5 g glucose per 4.5 g water
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What mass of trisodium phosphate is required to prepare 250.0 mL of a solution that is 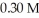 In sodium ion?
In sodium ion?
A) 37 g
B) 12 g
C) 7.7 g
D) 4.1 g
E) 3.0 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the oxidation number change for the manganese atom in the following unbalanced reduction half-reaction: Mn O4- (aq) + H+(aq) → Mn2+(aq) + H2O(l)
A) -7
B) -5
C) +5
D) +7
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction 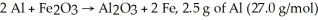 And 7.2 g of Fe2O3 (159.8 g/mol) produce how many g of Fe (55.9 g/mol) ?
And 7.2 g of Fe2O3 (159.8 g/mol) produce how many g of Fe (55.9 g/mol) ?
A) 2.5 (55.9/27.0) g
B) 2.5 (55.9) (2) /(27.0) (2) g
C) 7.2 (55.9) (2) /159.8 g
D) 7.2 (55.9/159.8) g
E) 2.5 (55.9/159.8) g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the reaction: 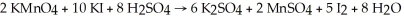 How many moles of K2SO4 are produced by allowing five moles each of KMnO4,KI,and H2SO4 to react?
How many moles of K2SO4 are produced by allowing five moles each of KMnO4,KI,and H2SO4 to react?
A) 3 mol
B) 1 mol
C) 2 mol
D) 4 mol
E) 5 mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
7.0 g of nitrogen is reacted with 5.0 g of hydrogen to produce ammonia according to the chemical equation shown below.Which one of the following statements is FALSE? N2(g) + 3 H2(g) → 2 NH3(g)
A) 3.5 g of hydrogen are left over.
B) Hydrogen is the excess reactant.
C) Nitrogen is the limiting reactant.
D) The theoretical yield of ammonia is 15 g.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 85.6 mL of a 6.75 M solution are diluted to 6.20 L with water,what is the concentration of the final solution?
A) 6.75 (6.20/85.6) M
B) 6.75 (8.56/6.20) M
C) 6.75 (6200/85.6) M
D) 6.75 (85.6/6200) M
E) 8.56 (6.20/6.75) M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following reactions: Fe + Br2 → FeBr2 3 FeBr2 + Br2 → Fe3Br8 If each reaction is 82.0% efficient,what mass of iron is necessary to make 8.45 g of Fe3Br8?
A) 0.870 g
B) 3.73 g
C) 2.14 g
D) 1.75 g
E) 2.61 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the percent yield if 185 grams of SiO2 are made from 328 g of Cr2O3 by the following equation? 3 Si(s) + 2 Cr2O3(s) → 3 SiO2(s) + 4 Cr(l)
A) 142%
B) 70%
C) 56%
D) 105%
E) 95%
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which metal will produce the most hydrogen per gram of metal?
A) 2 Li + 2 HCl → 2 LiCl + H2
B) Sn + 4 HCl → SnCl4 + 2 H2
C) 2 Fe + 6 HCl →2 FeCl3 + 3 H2
D) Mg + 2 HCl → MgCl2 + H2
E) 2 Cr + 6 HCl → 2 CrCl3 + 3H2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 8.52 g each of zinc,potassium dichromate,and sulfuric acid are reacted by the reaction: 4 Zn + K2Cr2O7 + 7 H2SO4→ 4 ZnSO4 + 2 CrSO4 +K2SO4 + 7 H2O How many grams of zinc will be left unreacted?
A) 0.94 g
B) 3.65 g
C) 5.27 g
D) 1.89 g
E) 3.25 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Cryolite is a compound needed for the Hall-Heroult process for producing aluminum.Cryolite is produced by the following reaction: 6 HF + Al(OH) 3 + 3 NaOH → Na3AlF6 + 6 H2O How many grams of cryolite are produced if the reaction has a 94.3% yield and a limiting reagent of 27.8 grams of HF?
A) 275 g
B) 48.6 g
C) 45.9 g
D) 15.0 g
E) 15.9 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following reactions: Fe + Br2 → FeBr2 3 FeBr2 + Br2 → Fe3Br8 If each reaction is 82.0% efficient,what mass of Fe3Br8 is produced from 1.00 g Fe?
A) 4.81 g
B) 3.94 g
C) 2.65 g
D) 3.24 g
E) 2.57 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of BCl3 are needed to produce 10.0 g of HCl(aq) in the following reaction? BCl3(g) + 3 H2O(l) → 3 HCl(aq) + B(OH) 3(aq)
A) 0.0914 mol
B) 0.274 mol
C) 0.823 mol
D) 10.9 mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of H3PO4 are produced when 20.0 g of HCl are produced by the reaction 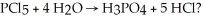
A) (20.0/36.5) g
B) (20.0/35.5) /5 g
C) (20.0/36.5) /5 g
D) (20.0/98.0) g
E) (20.0/98.0) /5 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molarity of 10.9 g KCl dissolved in 150.0 mL of water?
A) 0.0727 M
B) 0.146 M
C) 0.975 M
D) 0.0219 M
E) 0.667 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction 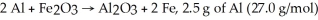 And 7.2 g of Fe2O3 (159.8 g/mol) produce 5.03 g of Fe (55.9 g/mol) .Calculate the extent of reaction ,x.
And 7.2 g of Fe2O3 (159.8 g/mol) produce 5.03 g of Fe (55.9 g/mol) .Calculate the extent of reaction ,x.
A) 0.362
B) 0.089
C) 0.045
D) 0.092
E) 0.405
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the concentration (M)of sodium ions in 4.57 L of a 0.398 mol L-1 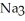 P(aq)solution?
P(aq)solution?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Aluminum metal reacts with aqueous iron(II) chloride to form aqueous aluminum chloride and iron metal.What is the stoichiometric coefficient for aluminum when the chemical equation is balanced using the lowest,whole-number stoichiometric coefficients?
A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 170
Related Exams