A) 7.1 × 10-8
B) 3.6 × 10-22
C) 3.6 × 10-1
D) 3.6 × 10-8
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hydroxide-ion concentration of a saturated solution of Ni(OH) 2? For Ni(OH) 2,Ksp = 2.0 × 10-15.
A) 2.8 × 10-3 M
B) 7.9 × 10-6 M
C) 1.0 × 10-7 M
D) 2.7 × 10-2 M
E) 1.6 × 10-5 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following equilibrium constants,
Zn(IO3) 2 Ksp = 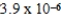 Zn(NH3) 42+ Kf =
Zn(NH3) 42+ Kf = 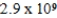
Determine Kc for the dissolution of the sparingly soluble salt Zn(IO3) 2 in aqueous ammonia (shown below) .
Zn(IO3) 2(s) + 4NH3(aq)
Determine Kc for the dissolution of the sparingly soluble salt Zn(IO3) 2 in aqueous ammonia (shown below) .
Zn(IO3) 2(s) + 4NH3(aq)  Zn(NH3) 42+(aq) + 2IO3-(aq)
Zn(NH3) 42+(aq) + 2IO3-(aq)
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the solubility product expression for Zn3(PO4) 2?
A) Ksp = [Zn32+][(PO43-) 2]
B) Ksp = [3Zn2+]3[2PO43-]2
C) Ksp = [Zn2+][2PO43-]
D) Ksp = [Zn3+]2[PO42-]3
E) Ksp = [Zn2+]3[PO43-]2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the maximum Sr2+ concentration possible in a solution that has a 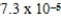 M sulfide-ion concentration without precipitating strontium sulfate? For SrSO4,Ksp = 2.5 × 10-7.
M sulfide-ion concentration without precipitating strontium sulfate? For SrSO4,Ksp = 2.5 × 10-7.
A) ![]() M
M
B) ![]() M
M
C) ![]() M
M
D) ![]() M
M
E) ![]() M
M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Suppose 50.00 mL of 2.0 × 10-6 M Fe(NO3) 3 is added to 50.00 mL of 2.0 ×10-4 M KIO3.Which of the following statements is true? For Fe(IO3) 3,Ksp = 1.0 × 10-14.
A) A precipitate forms because Qc > Ksp.
B) A precipitate forms because Qc < Ksp.
C) No precipitate forms because Qc < Ksp.
D) No precipitate forms because Qc = Ksp.
E) No precipitate forms because Qc > Ksp.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An aqueous solution of Ag(CN) 2− is made by combining 0.0100 moles AgNO3 with 1.00 mole NaCN and diluting to 1.000 L.What is the molar concentration of Ag+ in the solution?
Ag+(aq) + 2CN-(aq)  Ag(CN) 2-(aq) ; Kf = 5.6 × 1018
Ag(CN) 2-(aq) ; Kf = 5.6 × 1018
A) 1.9 × 10-21 M
B) 5.3 × 1020 M
C) 5.8 × 1016 M
D) 1.7 × 10-17 M
E) 0.010 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You have two salts,AgX and AgY,with very similar Ksp values.You know that Ka for HX is much greater than Ka for HY.Which statement will be true?
A) AgX and AgY are less soluble in acidic solution than in pure water.
B) AgX is more soluble in acidic solution.
C) AgX and AgY are equally soluble in acidic solution.
D) AgY is more soluble in acidic solution.
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following solutions would CaC2O4 have the highest molar solubility?
A) 0.01 M Na2C2O4
B) 0.01 M NaCl
C) 0.01 M HCl
D) 0.01 M Ca(NO3) 2
E) 0.01 M NaHC2O4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the solubility product expression for La2(CO3) 3?
A) Ksp = [2La3+]2[3CO32-]3
B) Ksp = [La2+]2[CO32-]3
C) Ksp = [2La3+]2[CO32-]3
D) Ksp = [2La3+][3CO32-]
E) Ksp = [La3+]2[CO32-]3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of CaF2 will dissolve in 3.0 L of 0.041 M NaF solution? (Ksp for CaF2 = 4.0 × 10-11)
A) 3.3 × 10-10
B) 2.4 × 10-8
C) 7.1 × 10-8
D) 7.9 × 10-9
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 430 mL of 1 × 10-4 M Ca(NO3) 2 is mixed with 430 mL of 1 × 10-4 M NaF,what will occur? For CaF2,Ksp = 3.4 × 10-11.
A) No precipitate will form.
B) Sodium nitrate will precipitate.
C) Calcium nitrate will precipitate.
D) Calcium fluoride will precipitate.
E) Sodium fluoride will precipitate.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following salts has the lowest molar solubility?
A) SrCO3 (Ksp = 9.3 × 10-10)
B) MnS (Ksp = 2.5 × 10-10)
C) BaF2 (Ksp = 1 × 10-6)
D) BaSO4 (Ksp = 1.1 × 10-10)
E) AgCl (Ksp = 1.8 × 10-10)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following solutions would silver(I) phosphate,Ag3PO4,be least soluble?
A) 0.10 M Na3PO4
B) 0.10 M AgNO3
C) 0.10 M Na2HPO4
D) 0.10 M HNO3
E) 0.10 M NaH2PO4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The best explanation for the dissolution of ZnS in dilute HCl is that
A) the zinc ion is amphoteric.
B) the sulfide ion concentration is decreased by the formation of H2S.
C) the solubility product of ZnCl2 is less than that of ZnS.
D) the zinc ion concentration is decreased by the formation of a chloro complex.
E) the sulfide ion concentration is decreased by oxidation to sulfur.
Correct Answer

verified
Correct Answer
verified
Showing 101 - 115 of 115
Related Exams