A) Ksp = [Pb4+][4IO3-]4
B) Ksp = [Pb4+][IO3-]
C) Ksp = [Pb][IO3]4
D) Ksp = [Pb4+][IO3-]4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Silver nitrate (AgNO3) is slowly added to a solution containing 0.100 M Br− and 0.050 M FeCN64− until a precipitate just forms.What is the molar concentration of Ag+ just as the precipitate forms? AgBr Ksp = 5.0 × 10-13 and Ag4FeCN6 Ksp = 8.5 × 10-45.
A) 2.0 × 10-11 M Ag+
B) 5.0 × 10-12 M Ag+
C) 1.0 × 10-11 M Ag+
D) 3.3 × 10-12 M Ag+
E) 1.7 × 10-43 M Ag+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the concentration of silver(I) ion in a saturated solution of silver(I) carbonate containing 0.0046 M Na2CO3? For Ag2CO3,Ksp = 8.6 × 10-12.
A) 6.0 × 10-4 M
B) 2.0 × 10-9 M
C) 8.0 × 10-9 M
D) 4.3 × 10-5 M
E) 8.0 �× 10-4 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction represents a step in the separation of which analytical group of cations? Hg22+(aq) + 2Cl-(aq) → Hg2Cl2(s)
A) Analytical Group II
B) Analytical Group I
C) Analytical Group V
D) Analytical Group IV
E) Analytical Group III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of these solutions would silver(I) carbonate have the lowest molar solubility? For silver(I) carbonate,Ksp = 8.5 × 10-12.
A) 0.03 M H2CO3
B) 0.1 M AgNO3
C) 0.01 M AgNO3
D) 0.1 M Na2CO3
E) pure water
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar solubility of lead(II) sulfate at 25°C? The solubility product constant for lead(II) sulfate is 1.7 × 10-8 at 25°C.
A) 1.7 × 10-8 M
B) 5.7 × 10-3 M
C) 8.5 × 10-9 M
D) 1.6 × 10-3 M
E) 1.3 × 10-4 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 370 mL of 1 × 10-8 M Al(NO3) 3 is mixed with 370 mL of 1 × 10-8 M NaOH,what will occur? For Al(OH) 3,Ksp = 4.6 × 10-33.
A) Aluminum hydroxide will precipitate.
B) Sodium hydroxide will precipitate.
C) Aluminum nitrate will precipitate.
D) Sodium nitrate will precipitate.
E) No precipitate will form.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the solubility product expression for mercury(I) chloride,Hg2Cl2?
A) Ksp = [Hg22+][2Cl-]2
B) Ksp = [Hg22+][Cl-]2
C) Ksp = [Hg22+][2Cl- ]
D) Ksp = [Hg2][Cl2]
E) Ksp = [Hg+]2[Cl-]2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hydroxide ion concentration of a saturated solution of Cu(OH) 2 is 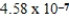 M.What is the solubility product constant for Cu(OH) 2?
M.What is the solubility product constant for Cu(OH) 2?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Figures I-IV represent ionic compounds formed upon the mixing of an aqueous solution containing cation C with an aqueous solution containing anion A.Identify the figure(s) that represent(s) products for which Ksp = 4s3,where s is the molar solubility of the ionic compound. 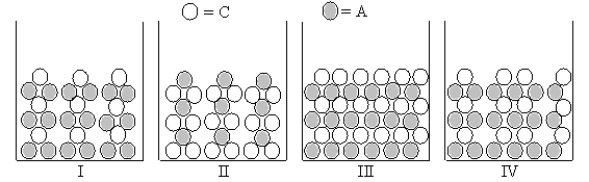
A) both I and II
B) only II
C) only IV
D) only I
E) only III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Solid KCN is added to a solution composed of 0.10 M Ag+ and 0.10 M Zn2+ just until a precipitate forms.What is the composition of this initial precipitate? AgCN Ksp = 2.2 × 10-16 and Zn(CN) 2 Ksp = 3 × 10-16.
A) The precipitate is pure AgCN(s) .
B) The precipitateis pure Zn(CN) 2(s) .
C) The precipitate is a mixture of AgCN(s) and Zn(CN) 2(s) .
D) The precipitate is a mixture of KCN(s) and AgCN(s) .
E) The precipitate is a mixture of KCN(s) and Zn(CN) 2(s) .
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar solubility of zinc hydroxide at pH 12.34? For Zn(OH) 2,Ksp = 2.1 × 10-16; for Zn(OH) 42-,Kf = 2.8 × 1015.
A) 1.2 × 10-25 M
B) 1.3 × 10-2 M
C) 2.8 × 10-4 M
D) 3.7 × 10-6 M
E) 1.4 × 10-8 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The concentration of barium carbonate in a saturated aqueous solution of the salt at 25°C is 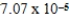 M.What is the Ksp of this sparingly soluble salt?
M.What is the Ksp of this sparingly soluble salt?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the solubility product expression for Al(OH) 3?
A) Ksp = [Al3+][3OH-]
B) Ksp = 3[Al3+][OH-]3
C) Ksp = [Al3+][OH-]3
D) Ksp = [Al3+][3OH-]3
E) Ksp = [Al3+][OH-]
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A _____ is an ion formed from a metal ion with a Lewis base attached to it by a coordinate covalent bond.
A) naive ion
B) radical ion
C) simple ion
D) monatomic ion
E) complex ion
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Suppose hydrogen sulfide is added to a solution that is 0.10 M in Cu2+,Pb2+,and Ni2+ such that the concentration of H2S is 0.10 M.When the pH of the solution is adjusted to 1.00,a precipitate forms.What is the composition of the precipitate?
H2S(aq) + 2H2O(l)  2H3O+(aq) + S2-(aq) ; Kc = 1.1 × 10-20
Salt
Ksp
CuS
6) 0 × 10-36
PbS
2) 5 × 10-27
NiS
3) 0 × 10-19
2H3O+(aq) + S2-(aq) ; Kc = 1.1 × 10-20
Salt
Ksp
CuS
6) 0 × 10-36
PbS
2) 5 × 10-27
NiS
3) 0 × 10-19
A) CuS only
B) PbS and NiS
C) CuS and PbS
D) NiS only
E) CuS,PbS,and NiS
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Suppose hydrogen sulfide is added to a solution that is 0.0010 M in Fe2+,Cd2+,Co2+,and Mn2+ such that the concentration of H2S is 0.10 M.When the pH of the solution is adjusted to 3,a precipitate forms.What is the composition of the precipitate?
H2S(aq) + 2H2O(l)  2H3O+(aq) + S2-(aq) ; Kc = 1.1 × 10-20
Salt
Ksp
FeS
6) 0 × 10-18
CdS
8) 0 × 10-27
CoS
4) 0 × 10-21
MnS
2) 5 × 10-10
2H3O+(aq) + S2-(aq) ; Kc = 1.1 × 10-20
Salt
Ksp
FeS
6) 0 × 10-18
CdS
8) 0 × 10-27
CoS
4) 0 × 10-21
MnS
2) 5 × 10-10
A) CdS only
B) CdS,CoS,FeS,and MnS
C) CdS,CoS,and FeS
D) CdS and FeS
E) CdS and CoS
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the two equilibria below,
Ag(NH3) 2+(aq)  Ag+(aq) + 2NH3(aq) ; Kd = 5.9 × 10-8AgI(s)
Ag+(aq) + 2NH3(aq) ; Kd = 5.9 × 10-8AgI(s)  Ag+(aq) + I−(aq) ; Ksp =
Ag+(aq) + I−(aq) ; Ksp = 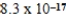 What is Kc for the following equilibrium?AgI(s) + 2NH3(aq)
What is Kc for the following equilibrium?AgI(s) + 2NH3(aq)  Ag(NH3) 2+(aq) + I-(aq)
Ag(NH3) 2+(aq) + I-(aq)
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not likely to form a complex ion with Al3+?
A) NH4+
B) NH3
C) OH−
D) H2O
E) CH3NH2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
After mixing an excess PbCl2 with a fixed amount of water,it is found that the equilibrium concentration of Pb2+ is 1.6 × 10-2 M.What is Ksp for PbCl2?
A) 4.0 × 10-6
B) 1.6 × 10-5
C) 2.5 × 10-4
D) 4.8 × 10-2
E) 1.0 × 10-6
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 115
Related Exams