A) K(s) + Br(g) + 3O(g) → KBrO3(s)
B) K(g) + Br(g) + 3O(g) → KBrO3(s)
C) K(g) + ![]() Br2(g) +
Br2(g) + ![]() O2(g) → KBrO3(s)
O2(g) → KBrO3(s)
D) K(s) + ![]() Br2(l) +
Br2(l) + ![]() O2(g) → KBrO3(s)
O2(g) → KBrO3(s)
E) K(s) + ![]() Br2(g) +
Br2(g) + ![]() O2(g) → KBrO3(s)
O2(g) → KBrO3(s)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the standard enthalpy change for the combustion of gaseous propylene,C3H6?
C3H6(g) + 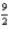 O2(g) → 3CO2(g) + 3H2O(l)
Substance
ΔH°f (kJ/mol)
C3H6(g)
+20) 4
CO2(g)
-393.5
H2O(l)
-285.8
O2(g) → 3CO2(g) + 3H2O(l)
Substance
ΔH°f (kJ/mol)
C3H6(g)
+20) 4
CO2(g)
-393.5
H2O(l)
-285.8
A) +2017.5 kJ
B) -2058.3 kJ
C) -658.9 kJ
D) -2017.5 kJ
E) +2058.3 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the kinetic energy of a 2000-lb car traveling at 59 miles per hour? (1 lb = 0.4536 kg,1 mi = 1.609 km)
A) 3.2 × 10-7 J
B) 1.5 × 106 J
C) 5.3 × 1019 J
D) 3.2 × 105 J
E) 4.7 × 10-8 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For which of the following equations is the enthalpy change at 1 atm pressure equal to the standard enthalpy of formation of liquid formic acid,HCOOH?
A) C(g) + 2H(g) + 2O(g) → HCOOH(l)
B) C(s) + 2H(g) + 2O(g) → HCOOH(l)
C) C(s) + H2(g) + O2(g) → HCOOH(l)
D) CO(g) + H2O(l) → HCOOH(l)
E) CO2(g) + H2(g) → HCOOH(l)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At constant pressure,the sign of q for the process CO2(s) → CO2(g) is expected to be
A) positive,and the process is exothermic.
B) negative,and the process is exothermic.
C) impossible to predict.
D) positive,and the process is endothermic.
E) negative,and the process is endothermic.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Two metals of equal mass with different heat capacities are subjected to the same amount of heat.Which undergoes the smaller change in temperature?
A) The metal with the higher heat capacity undergoes the smaller change in temperature.
B) Both undergo the same change in temperature.
C) You need to know the initial temperatures of the metals.
D) You need to know which metals you have.
E) The metal with the lower heat capacity undergoes the smaller change in temperature.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
From the following information,determine the enthalpy of formation of C2H4(g) . 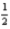 C2H4(g) → C(s) + H2(g) ; ΔH = -26.2 kJ
C2H4(g) → C(s) + H2(g) ; ΔH = -26.2 kJ
A) -26.2 kJ/mol
B) 26.2 kJ/mol
C) 104.8 kJ/mol
D) -52.4 kJ/mol
E) 52.4 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How much heat is evolved upon the complete oxidation of 6 g of aluminum at 25°C and 1 atm pressure? ( 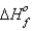 for Al2O3 is -1676 kJ/mol.)
4Al(s) + 3O2(g) → 2Al2O3(s)
for Al2O3 is -1676 kJ/mol.)
4Al(s) + 3O2(g) → 2Al2O3(s)
A) 9.238 × 103 kJ
B) 342.3 kJ
C) 684.7 kJ
D) 171.1 kJ
E) 85.59 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given:
4AlCl3(s) + 3O2(g) → 2Al2O3(s) + 6Cl2(g) ; ΔH = -529.0 kJ
Determine ΔH for the following thermochemical equation.
Cl2(g) +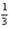 Al2O3(s) →
Al2O3(s) → 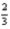 AlCl3(s) +
AlCl3(s) +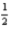 O2(g)
O2(g)
A) +264.5 kJ
B) +529.0 kJ
C) +88.2 kJ
D) +176.3 kJ
E) -176.3 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following changes at constant temperature and pressure: H2O(s) → H2O(l) ; ΔH1 H2O(l) → H2O(g) ; ΔH2 H2O(g) → H2O(s) ; ΔH3 Using Hess's law,the sum ΔH1 + ΔH2 + ΔH3 is
A) equal to zero.
B) sometimes greater than zero and sometimes less than zero.
C) less than zero.
D) cannot be determined without numerical values for ΔH.
E) greater than zero.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is incorrect?
A) Internal energy is a state function.
B) The value of q is positive when heat flows into a system from the surroundings.
C) The value of q is positive in an endothermic process.
D) Heat flows from a system into the surroundings in an endothermic process.
E) Enthalpy is a state function.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Exactly 209.2 J will raise the temperature of 10.0 g of a metal from 25.0°C to 60.0°C.What is the specific heat capacity of the metal?
A) 0.598 J/(g ∙ °C)
B) 1.67 J/(g ∙ °C)
C) 14.7 J/(g ∙ °C)
D) 50.0 J/(g ∙ °C)
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How much heat is liberated at constant pressure when 97.7 g of calcium oxide reacts with 29.0 L of carbon dioxide gas,measured at 1.00 atm pressure and 25.0°C? (R = 0.0821 L • atm/(K • mol) ) CaO(s) + CO2(g) → CaCO3(s) ; ΔH° = -178.3 kJ
A) -3.11 × 102 kJ
B) -1.74 × 104 kJ
C) -5.22 × 102 kJ
D) -2.11 × 102 kJ
E) -5.17 × 103 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The balanced equation representing the standard enthalpy of formation reaction for NH3(g) is
A) N(g) + ![]() H2(g) → NH3(g) .
H2(g) → NH3(g) .
B) ![]() N2(g) + 3H(g) → NH3(g) .
N2(g) + 3H(g) → NH3(g) .
C) N(g) + 3H(g) → NH3(g) .
D) ![]() N2(g) +
N2(g) + ![]() H2(g) → NH3(g) .
H2(g) → NH3(g) .
E) N2(g) + H2(g) → NH3(g) .
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is ΔH° for the following reaction? 2C2H2(g) + 5O2(g) → 4CO2(g) + 2H2O(l) Substance ΔH°f (kJ/mol) C2H2(g) +226.7 CO2(g) -393.5 H2O(l) -285.8
A) +1692.2 kJ
B) -452.6 kJ
C) -1692.2 kJ
D) +2599.0 kJ
E) -2599.0 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the thermochemical equation
2Al(s) + 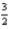 O2(g) → Al2O3(s) ; ΔH = -1676 kJ
Find ΔH for the following reaction.
2Al2O3(s) → 4Al(s) + 3O2(g)
O2(g) → Al2O3(s) ; ΔH = -1676 kJ
Find ΔH for the following reaction.
2Al2O3(s) → 4Al(s) + 3O2(g)
A) 838 kJ
B) 1676 kJ
C) -1676 kJ
D) 3352 kJ
E) -838 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The enthalpy change at 1 atm of which reaction corresponds to the standard enthalpy of formation of solid calcium nitrate,Ca(NO3) 2?
A) Ca2+(g) + 2NO3-(g) → Ca(NO3) 2(s)
B) Ca(s) + N2(g) + 3O2(g) → Ca(NO3) 2(s)
C) Ca(g) + 2N(g) + 6O(g) → Ca(NO3) 2(s)
D) Ca(s) + N2(g) + 2O3(g) → Ca(NO3) 2(s)
E) Ca2+(aq) + 2NO3-(aq) → Ca(NO3) 2(s)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 100 g sample of each of the following metals is heated from 35°C to 45°C.Which metal absorbs the lowest amount of heat energy? Metal Specific Heat Copper 0) 385 J/(g ∙ °C) Magnesium 1) 02 J/(g ∙ °C) Mercury 0) 138 J/(g ∙ °C) Silver 0) 237 J/(g ∙ °C) Lead 0) 129 J/(g ∙ °C)
A) lead
B) magnesium
C) silver
D) mercury
E) copper
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is false concerning the reaction of hydrogen gas and oxygen gas given below?
H2(g) + 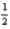 O2(g) → H2O(l) ; ΔH = -285.8 kJ
O2(g) → H2O(l) ; ΔH = -285.8 kJ
A) Per mole of O2,the change in enthalpy is -571.6 kJ.
B) The value -571.6 kJ pertains to 1 mol of liquid water.
C) If the equation is reversed,ΔH becomes +285.8 kJ.
D) If the equation is multiplied by 2,ΔH becomes -571.6 kJ.
E) For the reaction H2(g) + ![]() O2(g) → H2O(g) ,ΔH is not equal to -285.8 kJ.
O2(g) → H2O(g) ,ΔH is not equal to -285.8 kJ.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction of iron with hydrochloric acid is represented by the following thermochemical equation. Fe(s) + 2HCl(aq) → FeCl2(aq) + H2(g) ; ΔH° = -87.9 kJ How much heat is liberated at constant pressure if 0.154 g of iron reacts with 25.7 mL of 0.358 M HCl?
A) 1.85 kJ
B) 13.5 kJ
C) 0.242 kJ
D) 0.404 kJ
E) 87.9 kJ
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 119
Related Exams