A) alkali metals
B) alkaline earth metals
C) inner-transition metals
D) transition metals
Correct Answer

verified
Correct Answer
verified
Short Answer
Using shorthand notation,the ground-state electron configuration for Co2+ is predicted to be ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Both chlorine and bromine can be produced in the laboratory by reacting the halides with manganese(IV) oxide.If the following equation is balanced in an acidic solution,then the MnO2 is acting as a(n) ________ and the coefficient in front of H+ is ________. ____ MnO2(s) + ____ Cl-(aq) + ____ H+(aq) → ____ Mn2+(aq) + ____ Cl2(g) + ____ H2O(l)
A) oxidizing agent,2
B) oxidizing agent,3
C) oxidizing agent,4
D) reducing agent,3
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which element has the most favorable (most negative) electron affinity?
A) B
B) C
C) Li
D) N
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which ion has the smallest ionic radius?
A) Li+
B) Na+
C) K+
D) Rb+
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
An element that has the valence electron configuration 6s26p6 belongs to which period and group?
A) period 6;group 6A
B) period 6;group 8A
C) period 7;group 6A
D) period 7;group 8A
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following ionic compounds would be expected to have the highest lattice energy?
A) NaF
B) NaCl
C) NaBr
D) NaI
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which ionic compound would be expected to have the highest lattice energy?
A) Na2O
B) MgO
C) Al2O3
D) CO2
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which period 3 element has successive first through seventh ionization energies (kJ/mol) of Ei1 = 578;Ei2 = 1,817;Ei3 = 2,745;Ei4 = 11,575;Ei5 = 14,830;Ei6 = 18,376;and Ei7 = 23,293?
A) Mg
B) Al
C) S
D) Cl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Most of the compounds of the 2+ ions of the first row of the transition metals from Mn to Zn are colored due to absorption of visible light promoting an electron from one 3d orbital to another.Which of these ions should tend to form colorless compounds?
A) Mn2+
B) Co2+
C) Cu2+
D) Zn2+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element has the least favorable (least negative) electron affinity?
A) B
B) C
C) N
D) O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following three sets consist of atoms or ions with the same electron configuration in the ground state? (I) O2-,Ne,and Mg2+ (II) Ni,Cu+,and Zn2+ (III) Hg,Tl+,and Pb2+
A) all three sets
B) all but (I)
C) all but (II)
D) only (I)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the hydrogen halide acids is used to etch glass?
A) HF
B) HCl
C) HBr
D) HI
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name for the group of elements indicated by the shaded portion of the periodic table? 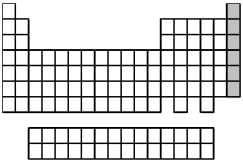
A) alkaline earth metals
B) group 3A elements
C) halogens
D) noble gases
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element has the most favorable (most negative) electron affinity?
A) Na
B) Mg
C) O
D) Ne
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The four spheres below represent Na+,Mg2+,F⁻,and O2-,not necessarily in that order. 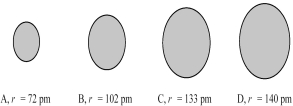 -Which sphere most likely represents the Mg2+ ion?
-Which sphere most likely represents the Mg2+ ion?
A) A
B) B
C) C
D) D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following,which element has the highest first ionization energy?
A) Ca
B) K
C) Li
D) Mg
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The four spheres below represent K+,Ca2+,Cl-,and S2-,not necessarily in that order. 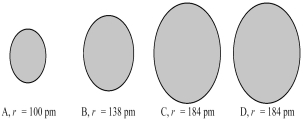 -Which sphere most likely represents the Cl⁻ ion?
-Which sphere most likely represents the Cl⁻ ion?
A) A
B) B
C) A or B
D) C or D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element,indicated by letter on the periodic table,is able to form compounds that do not obey the octet rule? 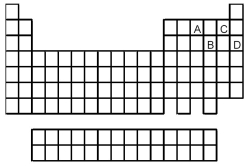
A) A
B) B
C) C
D) D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
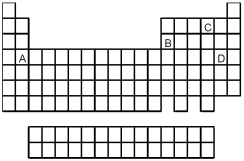 -What is the likely formula for the binary compound formed from the elements represented by letters A and C on the periodic table above?
-What is the likely formula for the binary compound formed from the elements represented by letters A and C on the periodic table above?
A) AC
B) A2C
C) AC2
D) A2C3
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 173
Related Exams