A) S-.
B) S 2-.
C) SO32-.
D) SO42-.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A beta particle is
A) ![]() .
.
B) ![]() .
.
C) ![]() .
.
D) ![]() .
.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
"Isotopes" are atoms with the same number of ________ but different number of ________.
A) electrons,protons
B) neutrons,protons
C) protons,electrons
D) protons,neutrons
Correct Answer

verified
Correct Answer
verified
Short Answer
Chlorine has two common isotopes,chlorine-35 and chlorine-37,and an atomic mass of 35.45 amu.The natural abundance of chlorine-35 is ________ (greater than,less than,the same as)the natural abundance of chlorine-37.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Beta decay of 32P produces a beta particle and
A) (28Al.)
B) (31P.)
C) (32Si.)
D) (32S.)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not explained by Dalton's atomic theory?
A) conservation of mass during a chemical reaction
B) the existence of more than one isotope of an element
C) the law of definite proportions
D) the law of multiple proportions
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The chemical formula for nitrous acid is
A) H3N(aq) .
B) H NO2(aq) .
C) H NO3(aq) .
D) H2N2O6(aq) .
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the following drawings,shaded spheres represent cations and unshaded spheres represent anions. 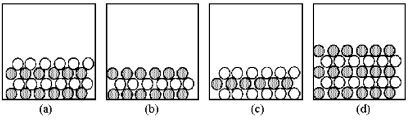 -Which drawing represents the ionic compound Mg3(PO4) 2?
-Which drawing represents the ionic compound Mg3(PO4) 2?
A) drawing (a)
B) drawing (b)
C) drawing (c)
D) drawing (d)
Correct Answer

verified
Correct Answer
verified
Short Answer
The charge to mass ratio of an electron was determined from Rutherford's cathode-ray tube experiment to be 1.759 × 108 C/g and the charge on a single electron was determined from the Millikan oil drop experiment to be 1.602 × 10-19 C,so the mass of a single electron is ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which are isotopes? An atom that has an atomic number of 20 and a mass number of 42 is an isotope of an atom that has
A) an atomic number of 21 and a mass number of 42.
B) an atomic number of 20 and a mass number of 40.
C) 22 neutrons and 20 protons.
D) 22 protons and 20 neutrons.
Correct Answer

verified
Correct Answer
verified
Short Answer
Nuclei that are in the band of stability have a neutron/proton ratio ________ (equal to,greater than,less than)1:1.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One mole of which element has the smallest mass?
A) Co
B) Cu
C) Ni
D) Zn
Correct Answer

verified
Correct Answer
verified
Multiple Choice
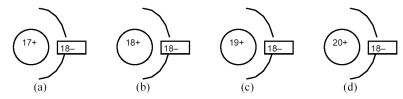 -Which of the above drawings represents a K+ ion?
-Which of the above drawings represents a K+ ion?
A) drawing (a)
B) drawing (b)
C) drawing (c)
D) drawing (d)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Tritium, 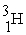 ,is formed in the upper atmosphere when
,is formed in the upper atmosphere when  Captures a neutron and then decays.What is the other product of this reaction?
Captures a neutron and then decays.What is the other product of this reaction?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Short Answer
The number of electrons in the ion C4- is ________.
Correct Answer

verified
Correct Answer
verified
Short Answer
The symbol for technetium-98 is ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the molecular formula corresponding to the following ball-and-stick molecular representation of vitamin C (ascorbic acid) (gray = C,unshaded = H,black = O) .In writing the formula,list the atoms in alphabetical order. 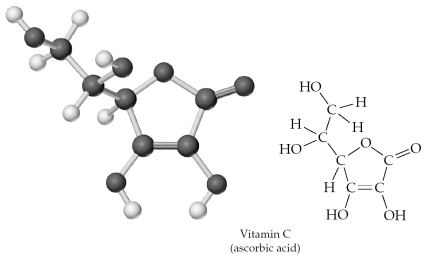
A) CHO
B) C3H4O3
C) C6H4O6
D) C6H8O6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Tell the type of decay process occurring in the following nuclear reaction. 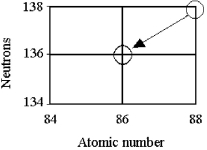
A) α emission
B) β emission
C) γ emission
D) electron capture or positron emission
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Boron-9 can be represented as
A) ![]() .
.
B) ![]() .
.
C) ![]() .
.
D) ![]() .
.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The definitive distinction between ionic bonding and covalent bonding is that
A) ionic bonding involves a sharing of electrons and covalent bonding involves a transfer of electrons.
B) ionic bonding involves a transfer of electrons and covalent bonding involves a sharing of electrons.
C) ionic bonding requires two nonmetals and covalent bonding requires a metal and a nonmetal.
D) covalent bonding requires two nonmetals and ionic bonding requires a metal and a nonmetal.
Correct Answer

verified
Correct Answer
verified
Showing 141 - 160 of 257
Related Exams