A) 3,010 kJ/mol
B) -687.6 kJ/mol
C) -277.6 kJ/mol
D) 687.6 kJ/mol
E) 1,367 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Methanol (CH3OH) burns according to the equation 2CH3OH(l) + 3O2(g) 2CO2(g) + 4H2O(l) , H°rxn = -1454 kJ/mol. How much heat, in kilojoules, is given off when 75.0 g of methanol is burned?
A) 727 kJ
B) 3.22 * 103 kJ
C) 1.45 * 103 kJ
D) 1.70 * 10-3 kJ
E) 3.41 * 103 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction C(graphite) + O2(g) CO2(g) H° = -393 kJ/mol How many grams of C(graphite) must be burned to release 275 kJ of heat?
A) 22.3 g
B) 0.70 g
C) 12.0 g
D) 17.1 g
E) 8.40 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Pentaborane B5H9(s) burns vigorously in O2 to give B2O3(s) and H2O(l) . Calculate H°rxn for the combustion of 1 mol of B5H9. 0H°f[B2O3(s) ] = -1,273.5 kJ/mol H°f[B5H9(s) ] = 73.2 kJ/mol H°f[H2O(l) ] = -285.8 kJ/mol
A) -1,2735 kJ/mol
B) -4,543 kJ/mol
C) -18,170 kJ/mol
D) -9,086 kJ/mol
E) -8,448 kJ/mol
Correct Answer

verified
Correct Answer
verified
Short Answer
A 26.2 g piece of copper metal is heated from 21.5°C to 201.6°C. Calculate the amount of heat absorbed by the metal. The specific heat of Cu is 0.385 J/g·°C.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The combustion of butane produces heat according to the equation 2C4H10(g) + 13O2(g) 0 8CO2(g) + 10H2O(l) H°rxn= -5,314 kJ/mol How many grams of butane must be burned to release 1.00 * 104 kJ of heat?
A) 30.9 g
B) 61.8 g
C) 109 g
D) 153 g
E) 219 g
Correct Answer

verified
Correct Answer
verified
Short Answer
The value of H°rxn for the following reaction is -6535 kJ/mol. 2C6H6(l)+ 15O2(g) 12CO2(g)+ 6H2O(g) How many kilojoules of heat will be evolved during the combustion of 16.0 g of C6H6(l)?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When 0.560 g of Na(s) reacts with excess F2(g) to form NaF(s) , 13.8 kJ of heat is evolved at standard-state conditions. What is the standard enthalpy of formation ( H°f) of NaF(s) ?
A) 24.8 kJ/mol
B) 570 kJ/mol
C) -24.8 kJ/mol
D) -7.8 kJ/mol
E) -570 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Potential energy is
A) the energy stored within the structural units of chemical substances.
B) the energy associated with the random motion of atoms and molecules.
C) solar energy, i.e. energy that comes from the sun.
D) energy available by virtue of an object's position.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Chemical energy is
A) the energy stored within the structural units of chemical substances.
B) the energy associated with the random motion of atoms and molecules.
C) solar energy, i.e. energy that comes from the sun.
D) energy available by virtue of an object's position.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At 25°C, the following heats of reaction are known: 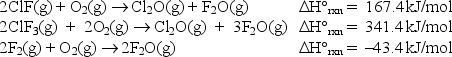 At the same temperature, use Hess's law to calculate H°rxn for the reaction: ClF(g) + F2(g) ClF3(g)
At the same temperature, use Hess's law to calculate H°rxn for the reaction: ClF(g) + F2(g) ClF3(g)
A) -217.5 kJ/mol
B) -130.2 kJ/mol
C) 217.5 kJ/mol
D) -108.7 kJ/mol
E) 465.4 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The combustion of pentane produces heat according to the equation C5H12(l) + 8O2(g) 5CO2(g) + 6H2O(l) H°rxn= -3,510 kJ/mol How many grams of CO2 are produced per 2.50 * 103 kJ of heat released?
A) 0.0809 g
B) 3.56 g
C) 31.3 g
D) 157 g
E) 309 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Solid sodium peroxide (Na2O2) reacts with liquid water yielding aqueous sodium hydroxide and oxygen gas. How much heat is released when 250.0 L of oxygen gas is produced from the reaction of sodium peroxide and water if the reaction is carried out in an open container at 1.000 atm pressure and 25°C? (Given: H°f[Na2O2(s) ] = -510.9 kJ/mol; H°f[NaOH(aq) ] = -469.2 kJ/mol; H°f[H2O(l) ] = -285.8 kJ/mol)
A) 35,400 kJ
B) 1740 kJ
C) 141.7 kJ
D) 3330 kJ
E) 2900 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Styrene, C8H8, is one of the substances used in the production of synthetic rubber. When styrene burns in oxygen to form carbon dioxide and liquid water under standard-state conditions at 25°C, 42.62 kJ are released per gram of styrene. Find the standard enthalpy of formation of styrene at 25°C. (Given: H°f[CO2(g) ] = -393.5 kJ/mol, H°f[H2O(l) ] = -285.8 kJ/mol, H°f[H2O(g) ] = -241.8 kJ/mol)
A) 323.8 kJ/mol
B) -4249 kJ/mol
C) -8730 kJ/mol
D) -636.7 kJ/mol
E) 147.8 kJ/mol
Correct Answer

verified
Correct Answer
verified
Short Answer
Find H°rxn for the reaction CH4(g)+ 2O2(g) CO2(g)+ 2H2O(l). [ H°f (CH4(g))= -74.8 kJ/mol; H°f (CO2(g))= -393.5 kJ/mol; H°f (H2O(l))= -285.5 kJ/mol]
Correct Answer

verified
Correct Answer
verified
True/False
The heat absorbed by a system at constant pressure is equal to E + P V.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given H2(g) + (1/2) O2(g) H2O(l) , H° = -286 kJ/mol, determine the standard enthalpy change for the reaction 2H2O(l) 2H2(g) + O2(g) .
A) ( H°) = -286 kJ/mol
B) ( H°) = +286 kJ/mol
C) ( H°) = -572 kJ/mol
D) ( H°) = +572 kJ/mol
E) ( H°) = -143 kJ/mol
Correct Answer

verified
Correct Answer
verified
Short Answer
A 0.3423 g sample of pentane, C5H12, was burned in a bomb calorimeter. The temperature of the calorimeter and the 1.000 kg of water contained therein rose from 20.22°C to 22.82°C. The heat capacity of the calorimeter is 2.21 kJ/°C. The heat capacity of water = 4.184 J/g·°C. What is the heat of combustion, in kilojoules, per gram of pentane?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas is allowed to expand, at constant temperature, from a volume of 1.0 L to 10.1 L against an external pressure of 0.50 atm. If the gas absorbs 250 J of heat from the surroundings, what are the values of q, w, and E? 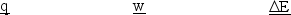
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
True/False
The heat capacity of 20.0 g of water is 83.7 J/°C.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 105
Related Exams