A) 6.5 L
B) 7.5 L
C) 8.5 L
D) 9.5 L
E) None of the above
Correct Answer

verified
Correct Answer
verified
True/False
At constant pressure, the density of a gas increases with an increase in temperature.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The temperature of a sample of argon gas in a 365 mL container at 740. mmHg and 25 C is lowered to 12 C. Assuming the volume of the container and the amount of gas is unchanged, calculate the new pressure of the argon.
A) 0.468 atm
B) 0.931 atm
C) 1.02 atm
D) 1.54 atm
E) 2.03 atm
Correct Answer

verified
Correct Answer
verified
True/False
An aerosol can with a volume of 0.50 L has a bursting point of 2.6 atm. If the can contains 1.0 g CO2 and is heated to 400 C, it will burst.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the number of moles of gas contained in a 10.0 L tank at 22 C and 105 atm. (R = 0.08206 L.atm/K.mol)
A) 1.71 * 10-3 mol
B) 0.0231 mol
C) 1.03 mol
D) 43.4 mol
E) 582 mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The van der Waals equation is a modification of the ideal gas equation. What two factors does this equation account for
A) (1) Real gas molecules exert forces on each other. (2) Gas molecules have energy.
B) (1) Real gas molecules exert ionic forces on each other. (2) Gas molecules have energy
C) (1) Real gas molecules exert forces on each other. (2) Gas molecules have volume.
D) (1) Real gas molecules exert ionic forces on each other. (2) Gas molecules have volume.
E) None of the above have BOTH of the two factors accurately stated
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Samples of the following volatile liquids are opened simultaneously at one end of a room. If you are standing at the opposite end of this room, which species would you smell first (Assume that your nose is equally sensitive to all these species.)
A) ethyl acetate (CH3COOC2H5)
B) camphor (C10H16O)
C) diethyl ether (C2H5OC2H5)
D) naphthalene (C10H8)
E) pentanethiol (C5H11SH)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Phosgene, a chemical warfare agent used in World War I, consists of 12.41% C, 16.17% O, and 71.69% Cl. 1.00 L of this gas at STP has a mass of 4.42 g. What is the molecular formula of phosgene
A) COCl
B) C2OCl2
C) COCl3
D) COCl2
E) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement is false
A) The average kinetic energies of molecules from samples of different "ideal" gases are the same at the same temperature.
B) The molecules of an ideal gas are relatively far apart.
C) All molecules of an ideal gas have the same kinetic energy at constant temperature.
D) Molecules of a gas undergo many collisions with each other and the container walls.
E) Molecules of greater mass have a lower average speed than those of less mass at the same temperature.
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
What is the molar mass of Freon-11 gas if its density is 6.13 g/L at STP
A) 0.274 g/mol
B) 3.64 g/mol
C) 78.2 g/mol
D) 137 g/mol
E) 365 g/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 0.271 g sample of an unknown vapor occupies 294 mL at 140. C and 847 mmHg. The empirical formula of the compound is CH2. What is the molecular formula of the compound
A) CH2
B) C2H4
C) C3H6
D) C4H8
E) C6H12
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
What is the pressure (in atmospheres) of the sample of gas trapped in the closed-tube mercury manometer shown below if h = 23.6 cm 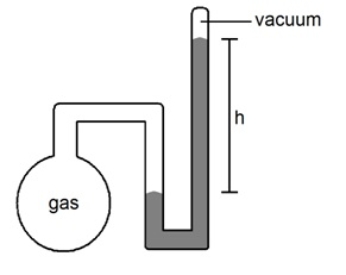
A) 0.211 atm
B) 0.311 atm
C) 0.411atm
D) 0.511atm
E) None of the above
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which one of the following is not an example of an element that occurs as a gas at room temperature and pressure
A) Helium
B) Neon
C) Chlorine
D) Bromine
E) Nitrogen
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What volume of sulfur dioxide gas at 45 C and 723 mmHg will react completely with 0.870 L of oxygen gas at constant temperature and pressure? 2 SO2(g) + O2(g) 2SO3(g)
A) 0.0317 L
B) 0.0634 L
C) 0.870 L
D) 1.74 L
E) 3.48 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What will happen to the height (h) of the column of mercury in the manometer shown below if the stopcock is opened 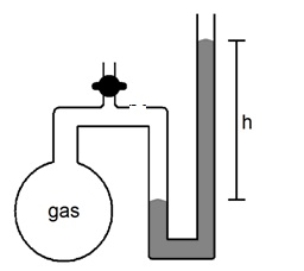
A) h will decrease
B) h will not change
C) h will increase
D) not enough information given to answer the question
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mole fraction of oxygen molecules in dry air is 0.2095. What volume of dry air at 1.00 atm and 25 C is required for burning 1.00 L of octane (C8H18, density = 0.7025 g/mL) completely, yielding carbon dioxide and water
A) 150 L
B) 367 L
C) 718 L
D) 1880 L
E) 8970 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When active metals such as magnesium are immersed in acid solution, hydrogen gas is evolved. Calculate the volume of H2(g) at 30.1 C and 0.85 atm that can be formed when 275 mL of 0.725 M HCl solution reacts with excess Mg to give hydrogen gas and aqueous magnesium chloride.
A) 3.4 * 10-3 L
B) 2.2 L
C) 2.9 L
D) 5.8 L
E) 11.7 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molar mass of a gaseous substance if 0.125 g of the gas occupies 93.3 mL at STP.
A) 30.0 g/mol
B) 30.2 g/mol
C) 30.4g/mol
D) 30.6 g/mol
E) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the pressure of a gas sample is tripled and the absolute temperature is quadrupled, by what factor will the volume of the sample change
A) 12
B) 4/3
C) 3/4
D) 1/3
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If equal masses of O2(g) and HBr(g) are in separate containers of equal volume and temperature, which one of these statements is true
A) The pressure in the O2 container is greater than that in the HBr container.
B) There are more HBr molecules than O2 molecules.
C) The average velocity of the O2 molecules is less than that of the HBr molecules.
D) The average kinetic energy of HBr molecules is greater than that of O2 molecules.
E) The pressures of both gases are the same.
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 130
Related Exams