A) Dispersion and Dipole-dipole
B) Dipole-dipole and Ionic
C) Ion-dipole and Hydrogen bonding
D) Hydrogen bonding and Dispersion
E) Dispersion
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the amount of enthalpy required to heat 25.0 g of solid benzene (C6H6) at -10 C to liquid benzene at 20.0 C. Thermodynamic data for benzene: specific heat of solid benzene = 1.52 J/g· C; specific heat of liquid benzene = 1.73 J/g· C; melting point = 5.5 C; Hfus = 9.9 kJ/mol.
A) 3.8 kJ
B) 4.1 kJ
C) 4.4 kJ
D) 4.7 kJ
E) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Indicate all the types of intermolecular forces of attraction in SF4(g) .
A) Dispersion and Dipole-dipole
B) Dipole-dipole and Ionic
C) Ion-dipole and Hydrogen bonding
D) Hydrogen bonding and Dispersion
E) Dispersion
Correct Answer

verified
Correct Answer
verified
Multiple Choice
BaCl2 crystallizes such that the Ba2+ ions are in a face-centered cubic arrangement and the Cl- ions are in the holes of the lattice (fluorite structure) . How many Cl- ions are present in one unit cell of this crystal
A) 1
B) 2
C) 4
D) 6
E) 8
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following crystallizes in a metallic lattice
A) C
B) NaMnO4
C) K
D) LiClO4
E) K2Cr2O7
Correct Answer

verified
Correct Answer
verified
True/False
The liquid phase exists at the point labeled b. 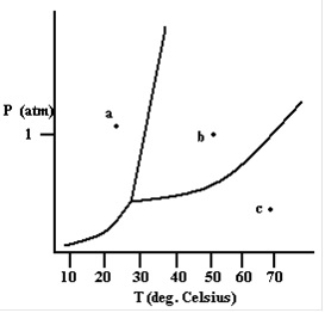
Correct Answer

verified
Correct Answer
verified
True/False
All intermolecular forces must be overcome in order for a substance to undergo a phase change from a solid to a liquid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Iron crystallizes in a body-centered cubic unit. The edge of this cell is 287 pm. What is the density of iron
A) 7.86 g/cm3
B) 7.66 g/cm3
C) 7.46 g/cm3
D) 7.26 g/cm3
E) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Suppose the atoms in a two-dimensional crystal have the following arrangement: 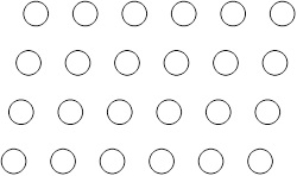 What is the coordination number of each atom in this crystal
What is the coordination number of each atom in this crystal
A) Two
B) Four
C) Six
D) Eight
E) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of following can form hydrogen bonds with water molecules (1) Na+ (2) CH3COOH (3) C2H6 (4) CH3NH2
A) (1) and (2)
B) (1) and (3)
C) (2) and (3)
D) (2) and (4)
E) (3) and (4)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
MgO has the same crystal structure as NaCl, face-centered cubic. How many oxide ions surround each Mg2+ ion as nearest neighbors
A) 4
B) 6
C) 8
D) 10
E) 12
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not true with regard to water
A) Water has a high heat capacity.
B) Water has an unusually high boiling point.
C) Water can form hydrogen bonds.
D) Ice is more dense than liquid water.
E) Water is a polar molecule.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The vapor pressure of a liquid in a closed container depends upon
A) the amount of liquid.
B) the surface area of the liquid.
C) the volume of the container.
D) the temperature.
E) none of the above.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the amount of heat that must be absorbed by 10.0 g of ice at -20 C to convert it to liquid water at 60.0 C. Given: specific heat (ice) = 2.1 J/g· C; specific heat (water) = 4.18 J/g· C; Hfus = 6.0 kJ/mol.
A) 63 kJ
B) 7.5 J
C) 420 J
D) 2,900 J
E) 6,300 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following substances is expected to have the highest boiling point
A) Br2
B) Cl2
C) F2
D) I2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on the phase diagram shown below, how will the melting point of the substance change if the pressure is increased above 1 atm 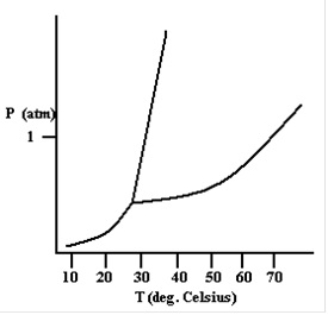
A) The melting point will decrease.
B) The melting point will remain the same.
C) The melting point will increase.
D) The substance will not melt at pressures of 1 atm and above; instead, the solid sublimes to form the gas phase.
Correct Answer

verified
Correct Answer
verified
True/False
Suppose the atoms in a two-dimensional crystal have the following arrangement: 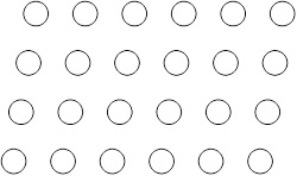 How many atoms are in one unit cell
A) One
B) Two
C) Three
D) Four
E) None of the above
How many atoms are in one unit cell
A) One
B) Two
C) Three
D) Four
E) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
W(s) is classified as a/an
A) metallic crystal.
B) covalent solid.
C) molecular crystal.
D) amorphous solid.
E) ionic crystal.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would be expected to have the highest vapor pressure at room temperature
A) ethanol, bp = 78 C
B) methanol, bp = 65 C
C) water, bp = 100 C
D) acetone, bp = 56 C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molar enthalpy of vaporization of carbon disulfide is 26.74 kJ/mol, and its normal boiling point is 46 C. What is the vapor pressure of CS2 at 0 C
A) 4160 torr
B) 447 torr
C) 313 torr
D) 139 torr
E) 5.47 torr
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 149
Related Exams